Review Article - Volume 1 Issue 1
Medium and Long-term Consequences of COVID-19
Carlos Remolina1*; Wdsving Osorio2
1Chief of Pulmonary Medicine at Trinitas Regional Medical Center/Rutgers New Jersey Medical School.
2Medical Intern Pavia Yauco Hospital, Puerto Rico.
Received Date : Nov 06, 2021
Accepted Date : Dec 07, 2021
Published Date: Dec 13, 2021
Copyright:© Carlos Remolina 2021.
*Corresponding Author : Carlos Remolina, Chief of Pulmonary Medicine at Trinitas Regional Medical Center/Rutgers New Jersey Medical School.
Email: carlos_remolina@yahoo.com
DOI: Doi.org/10.55920/2771-019X/1020
Abstract
Objective: To investigate the medium and long-term consequences of the COVID-19 disease and its impact on the functional capacity of patients. A clinical evaluation of the incidence of Fibromyalgia Syndrome (FMS) among COVID-19 survivors.
Methods: Retrospective study of 30 patients confirmed with COVID-19 who were evaluated at our pulmonary office in Linden, NJ, 6 months after the onset of symptoms. The 30 patient consisted of 18 women and 12 men with a mean (SD) age of 55 (10.7) years. Hospital admitted 13 (43%) patients, length of hospital stay 16 (5.85) days, 3 or more post-COVID symptoms in all patients. The evaluation included the following: lung volumes (total lung capacity (TLC), vital capacity (VC), functional residual capacity (FRC), residual volume (RV)), spirometry (forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and the ratio of these values FEV1/FVC), diffusion capacity of the lungs for carbon monoxide (DLCO), 6-minute walk test (6MWT), chest radiographs, CT of chest, musculoskeletal evaluation (hand grip strength capacity) and health related quality of life (HRQoL) by Short Form-36 Questionnaire (SF-36).
Results: The most relevant post-COVID symptoms were sleep disturbance 97%, shortness of breath 90%, chest pain 63%, post-nasal drip 60%, cough 21% and back pain 20%. The pulmonary function test performed on 16 survivors revealed 10 (62.5%), 12 (75%), 8 (50%) and 11 patients (69%) were < 80% of predicted values of the FVC, FEV1, TLC, and DLCO respectively. The CT of the chest showed that the ground-glass opacity (GGO) and the consolidations were persistent in 7 and 3 subjects, respectively. The 6MWT distance for 15 women was 270 (154.51) m, and for 10 men was 266 (144.67) m. The HGSC for 10 women was 11 (3.31) kilogram-force (kgf) for the right hand and 10 (4.14) kgf for the left hand. The HGSC for 6 men was 28 (16.99) kgf for the right hand and 26 (16.12) kgf for the left hand. There were 15 patients assessed through the SF-36 survey, showing that the most affected domains were the general health (GH), role physical (RP), mental health (MH), and energy/fatigue (EF). Twenty-seven patients (80%) of our population showed more than 6 multisite pain (MSP) locations, more usually at the chest, upper back, lower back and limbs associated with fatigue and sleep disturbance for at least 3 months.
Conclusion: This study has shown a significant reduction in the functional capacity and HRQoL of patients after COVID-19 infection. These chronic post-COVID symptoms were similar to those found in patients with Chronic Fatigue Syndrome/Fibromyalgia. We recommended our patient to initiate an early rehabilitation program called COVID-19 rehabilitation exercise modalities (CREM), to mitigate the Post-acute COVID-19 syndrome (PACS)
Keywords: COVID-19; SARS-CoV-2; fibromyalgia; functional capacity; pulmonary rehabilitation; PACS.
Introduction
Over the last several months, the U.S. healthcare system has challenged the consequences of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) outbreak. After the acute phase of the disease, patients return to our clinic reporting ongoing symptoms such as: chest pain, back pain, fatigue, myalgias, cough, post-nasal drip, shortness of breath, sleep fragmentation, memory loss, anxiety, and depression. As a result, patients have demonstrated inability to return to a regular pattern of activity along with a reduction in their physical capacity that negatively affects their overall performance at work. Therefore, the question arises as to how we can classify those patients who continue to exhibit symptoms of Coronavirus Disease 2019 (COVID-19) after their initial phase, and what follow up protocols we should use to further support them [1, 2].
Multiple risk factors correlate with the outcome of the disease, and need to be established during our first encounter with these patients. The two most important factors of this acquisition include their prior immune status and the viral load level of COVID-19 that each individual patient had exposed. Those two aspects will generate the “Cytokine Storm”, an exaggerated immune response from the host to the SARSCoV-2 infection [3]. Other important risk factors include age and preexisting comorbidities such as hypertension, coronary artery disease, congestive heart failure, diabetes mellitus, asthma, chronic obstructive pulmonary disease, pulmonary fibrosis and cancer [4, 5]. Studies so far suggest that 15 - 20% of patients with multiple comorbidities who were infected with SARS-CoV-2 develop severe respiratory syndrome or septic shock [6]. The mortality rate for COVID-19 has been reported three times higher in patients with diabetes mellitus and twoand-a-half times higher in patients with hypertension [7].
In our practice we observed that the majority of patients complained of easy fatigability, weakness, multiple body aches, shortness of breath and sleep disturbance, symptoms very similar to those present by patients with Fibromyalgia (FM). After the SARS-CoV pandemic in 2003, Modolfosky et al [8], found a connection between chronic pain, fatigue, psychological distress and fragmented sleep in a group of healthcare professionals that resulted in their failure to work productively at least a year after the diagnosis of this acute illness. Fibromyalgia is a chronic and often debilitating disorder characterized by widespread musculoskeletal pain. Other FM symptoms commonly include tenderness, stiffness, fatigue, sleep fragmentation, mood disturbance and reports of worsening symptoms following mild exertion [9]. Several studies on FM have shown that an insufficient muscle repair process after a viral process and impaired anti-inflammatory mechanism blunted by immune exhaustion, would result in worsening muscle pain, stiffness, and fatigue [10].
Our primary objective in this study was to identify the medium and long-term consequences of the COVID-19 and the impact it has on patient functional capacities. Furthermore, we evaluated the incidence of Fibromyalgia in patients who had been exposed to SARS-CoV2, compared with the finding in patients who suffered the SARS-CoV infection in 2003 [11, 12, 13] (Figure 1).
COVID-19 positive patients should always be evaluated by a Primary Care Physician (PCP), to determine the severity of their symptoms and the next step on their medical treatment. Patients with mild to moderate symptoms are sent home with an appropriate treatment plan and follow up. Additionally, patients with severe symptoms should be evaluated at Emergency Department (ED), for their high risk of developing COVID-19 pneumonia.
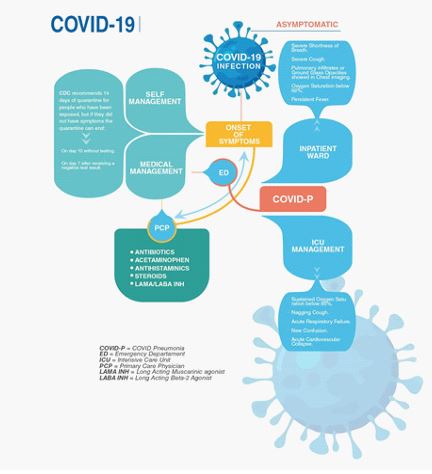
Figure 1: COVID-19 Infection Roadmap for PCP.
Methods
Participants
From March 1 to April 31, 2020, 30 patients with a positive reverse transcriptase-polymerase chain reaction (rRT-PCR) test for SARS-CoV2, were followed up for 6 months to evaluate the medium and long-term consequences of COVID-19 infection. There were 18 women and 12 men with a mean (SD) age of 55 (10.7) years. This retrospective study evaluated 30 patients with SARS-CoV2 at our local hospitals (Trinitas Regional Medical Center, Robert Wood Johnson University Hospital, Newark Beth Israel Medical Center, Overlook Medical Center and Rahway), after surviving the major outbreak during the months of March and April of 2020. Hospital admitted 13 (43%) patients, length of hospital stay 16 (5.85) days, 3 or more postCOVID symptoms in all patients. These patients complained of easy fatigability associated with musculoskeletal weakness and joint pain that interfered with their regular activities and reduced their work capacity.
Assessment
All patients were followed up within the pulmonary practice for 6 months after they were discharged from the hospital or after a partial recovery following the disease onset. During each visit, subjects were interviewed about post-COVID symptoms such as chest pain, back pain, fatigue, myalgias, cough, postnasal drip, shortness of breath, sleep fragmentation, memory loss, anxiety, and depression. In addition, each patient underwent a clinical evaluation for fibromyalgia points, pulmonary function testing, chest radiographs, CT of chest, 6-minute walk test (6MWT), musculoskeletal evaluation (Hand Grip Strength Capacity) and completed the medical outcomes SF-36 survey to measure Health Related Quality of Life (HRQoL).
Fibromyalgia Evaluation
In 2019, the Analgesic Anesthetic and Addiction Clinical Trial Translations Innovations Opportunities Networks (ACTTION) in association with the U.S. Food and Drug Administration (FDA) and the American Pain Society (APS), initiated the ACTTION-APS FM taxonomy to provide an evidence-based diagnostic system for FM. This approach allows us to diagnose FM in our population. All patients with FM would have multisite pain (MSP) in at least 6 sites of the total possible locations (head, right arm, left arm, chest, abdomen, upper back and spine, lower back and spine including buttocks, right leg and left leg), and a moderate to severe sleep problem or fatigue. Additionally, the MSP plus fatigue or sleep problems must have been present for at least 3 months [14].
Pulmonary Function Test (PFT)
An Easyone Pro Spirometry device was used to measure patient lung volumes of each individual subject such as total lung capacity (TLC), vital capacity (VC), functional residual capacity (FRC) and residual volume (RV). A spirometry test (pre and post bronchodilator) was conducted to obtain forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1/FVC ratio, and the diffusion capacity of the lungs for carbon monoxide (DLCO). These tests were achieved following the standards of the American Thoracic Society.
Radiographic Manifestations
Patients with a positive rRT-PCR test and clinical evidence of a viral pneumonia, were evaluated first with an anteroposterior projection of the chest, using an X-ray unit following the protocols of each medical institution. Several radiographic studies [15, 16], found that during the initial diagnosis of COVID-19, the chest X-rays sensitivity ranged only between 33-69%, suggesting that chest X-rays are not recommended as a screen method in COVID pneumonia. However, the CT of Chest was found to be more sensitive than rRT-PCR in confirming the diagnosis, with a 98% of sensitivity rate.
The radiographic manifestations of COVID pneumonia included multifocal and bilateral ground glass opacities (GGO) and consolidation with peripheral and basal predominance. The GGO is the early manifestation and it is defined as a hazy hyperattenuated area without obscuration of the underlying vessels, caused by partial filling of the alveolar spaces. The consolidation usually appeared 10 to 12 days after the GGO, and will often be seen organized altogether during the course of the disease. Other less common presentations were septal thickening, bronchiectasis, pleural effusion, thickening of the adjacent pleura, bronchovascular bundle distortion, lymphadenopathy and cavitation [17].
With an Image 20/20 Digital X-ray System under the American College of Radiology X-ray performance standards, patients identified with COVID pneumonia during their hospitalization completed a serial follow up chest X-rays to evaluate the progression, consistency, and improvement of their lung damage after recovery.
6 Minutes Walk Test (6MWT)
In 2002, The American Thoracic Society introduced the 6MWT as a submaximal level of functional capacity that reflects the exercise level that each individual needs to perform their activities of daily living. The 6MWT is used to measure the distances that a patient can walk along a 30 meters (flat surface) hallway in 6 minutes. It has been designated to measure functional capacity, as well as a predictor of morbidity and mortality in patients with moderate to severe heart or pulmonary disease. The age, height, weight and sex are undependable variables that needs to be in consideration during the interpretation of the 6MWT [18].
Oliveira et al [19] conducted an observational prospective study in Porto in 2018 on a population of 158 healthy subjects between 18 to 70 years old to establish reference equations and predict the correction of the undependable variables during the analysis of the 6MWT. They determined that 6MWT decreases 1.6 meters per year of age, 4.0 per unit of BMI, and increases 0.892 meters per beat per minute. Furthermore, on average males walk 58.4 meters more than females (p<0.001). In our study the 6MWT data sets were corrected with the Oliveira equation for an accurate presentation.
6MWT= Distance(m) - 1.6 x Age - 4.0 x BMI + 0.9 x HR + 58.4 x Sex
Musculoskeletal evaluation (Hand Grip Strength Capacity)
The Hand Grip Strength Capacity (HGSC) has been utilized to estimate the musculoskeletal performance in patients that require an appropriate evaluation of their functional capacity. HGSC has been considered a good index of overall muscle strength and it will be depleted in patients with Chronic Obstructive Pulmonary Disease (COPD)[20]. We used a Jamar® hydraulic hand dynamometer to take five bilateral measurements to each patient and the average value was compared with the normative grip strength data provided by Jamar® company.
Health Related Quality of Life (HRQoL)
The Short Form-36 (SF-36) is a scale used to evaluate the Health Related Quality of Life (HRQoL) status in a population. This tool is utilized for comparing the effect of various health conditions on the quality of life of a patient, without being associated with any specific disease. The SF-36 form evaluated 8 domains through 36 questions to assess the physical, mental and social welfare of each subject. Those domains are: physical functioning (PF), role -physical (RP), role-emotional (RE), bodily pain (BP), social functioning (SF), general health (GH), mental health (MH) and energy/fatigue (EF). A score for each domain ranges from 0 to 100, with the lowest scores (floor scores) indicating the more disable status. Scale scores were calculated using the SF-36 OrthoToolKit on the web, developed by Dr. Cathay Sherbourne [21]. She specializes in health outcomes measures in adults and children.
Statistical Data Analysis
Data analysis was carried out using Google spreadsheet with descriptive statistics. Mean (and SD) and 95% Confidence of Interval (95% CI) were used to present average values for normal dat
Results
The mean age for the group was 55 (SD,10.7) (range, 28 to 85 years of age) and of the 30 patients, 60% were women. Thirteen patients were hospitalized, and the mean length of stay was 16.1 (SD, 5.85) days. Three patients (10%) received non-invasive ventilation and 2 patients (7%) were intubated. Each individual patient was assessed for a mean of 2 (SD, 1.09) visits over the next 6 months after the onset of their COVID-19 symptoms. The majority of those patients were evaluated within the first month after their hospital discharge. Many patients presented symptoms related to their previous COVID infection over a mean of 138 (95% CI 133 to 143) days. The symptoms were frequently seen in all 30 (100%) patients. Furthermore, chest pain was reported in 19 (63%) patients and back pain in 6 (20%) patients. While 21 (70%) of those patients presented cough symptoms, it was described as intermittent in 3 (10%), persistent/dry in 11 (37%), persistent/white in 1 (3%), white in 3 (10%), and chronic in 3 (10%) patients. From those 21 patients with cough, 11 (33%) were former smokers and 2 (7%) were current smokers. Post-nasal drip (PND) and sialorrhea were symptoms frequently present in the study. We observed 18 (60%) patients with PND alone, 2 (7%) patients with sialorrhea alone, and 2 (7%) patients with both PND and sialorrhea. Exactly 27 (90%) patients reported shortness of breath on exertion and 2 (7%) were on oxygen therapy at the moment of their evaluation. Another observed symptom was sleep disturbance, which affected 29 (97%) patients, characterized by snoring in 16 (53%) patients, excessive daytime sleepiness in 10 (33%) patients, sleep fragmentation/ decreased total sleep time in 8 (27%) patients and obstructive sleep apnea in 9 (30%) patients.
The principal comorbidities observed in the study were cardiovascular in 16 (53%) patients divided as hypertension in 14 (47%), chronic atrial fibrillation in 3 (9%), coronary artery disease in 2 (7%), congestive heart failure in 1 (3%) and pulmonary embolism in 1 (3%) patients. Endocrine comorbidities were observed in 23 (77%) patients, characterized by obesity in 15 (50%), diabetes mellitus type 2 in 7 (23%), diabetes mellitus type 1 in 1 (3%), hypothyroidism in 4 (13%) and pancreatic cancer in 1 (3%) patients. Respiratory comorbidities were observed in 6 (20%) patients, characterized by chronic rhinitis in 2 (7%), asthma in 2 (7%), chronic obstructive pulmonary disease in 1 (3%), and pneumothorax in 1 (3%) patients. Musculoskeletal comorbidities were observed in 4 (13%) patients, characterized by rheumatoid arthritis in 2 (7%) patients, systemic lupus erythematosus in 1 (3%) patient and fibromyalgia in 1 (3%) patient. Renal comorbidities were observed in 3 (10%) patients, characterized by end stage renal disease in 2 (7%) patients and acute kidney disease in 1 (3%) patient.
Physical Examination (PE)
During their PE, none of the patients presented fever or any other symptoms of acute illness. All patients reported were not able to return to their normal activities within the first month after the onset of their COVID-19 infection. There were 6 (20%) patients treated with hydroxychloroquine during their acute phase. The average systolic blood pressure observed was 124 mmHg (95% CI 117 to 131). The average diastolic blood pressure observed was 74 mmHg (95% CI 71.5 to 76.5). The average Body Mass Index obtained was 31.3 (95% CI 28.8 to 33.8). The average pulse was 79.27 beats per minute, and the average oxygen saturation was 97.03% on rest. Exactly 29 patients (97%) showed over 6 sites of pain, located at bilateral elbows, chest, upper back, hips and bilateral knee. One patient showed a left upper weakness and another was noted with alopecia during the examination. Two patients suffered shingles throughout their COVID-19 recovery. One patient had a severe eosinophilia observed in the course of her hospitalization. Only one patient was sent to pulmonary rehabilitation after hospital discharge. Most patients were educated about pulmonary and physical rehabilitation at our clinic.
Pulmonary Function Test
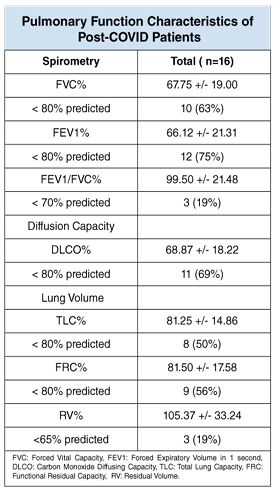
There were 16 (53%) patients that performed a pulmonary function test, while the others were excluded due to their symptoms or incapacity to perform the test. The FVC percentage was reduced from the predicted value in 10 (63%) patients, FEV1 percentage was reduced from the predicted value in 12 (75%) patients and the FEV1/ FVC percentage was reduced from the normative value in 3 (19%) patients. Abnormalities in the Diffusion Lung Capacity (DLCO) were observed in 11 (37%) patients, being lower in the more severe pneumonia cases. The lung values were also affected in those survivor patients, such as the TLC and FRC percentages, which were reduced from the normal value in 8 (50%) and 9 (56%) patients respectively. The RV percentage was observed under the normative value in 3 (19%) patients (Table 1).
Radiographic Manifestations
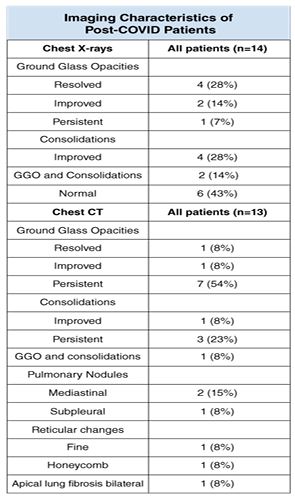
A total of 14 chest X-rays were performed, as follow ups, in patients with previous abnormal chest X-rays in their acute phase of COVID-19. Those images were obtained 3 to 6 weeks after onset of COVID-19 symptoms. Ground glass opacities (GGO) were the most common abnormality patterns observed on the chest X-rays. In 4 (28%) patients, the abnormalities were resolved, in 2 (14%) patients they were improved, and in 1 (7%) patient GGO was persistent at the peripheral location of the left lower lung. The second most common abnormality was the consolidation that was improved in 4 (28%) patients. They observed GGO changes and consolidation together that improved in 2 (14%) patients. Six (43%) patients were presented with no chest X-rays abnormalities.
Exactly 13 Chest CTs were performed on 7 patients with previously abnormal Chest CTs and on 6 patients with abnormal chest X-rays and pulmonary function tests. GGO appeared to be the most common finding seen in 7 (54%) patients bilaterally and at the peripheral locations, in one (8%) patient the GGO findings were improved, and in another patient those findings were resolved. The consolidation was observed in 3 (23%) patients bilaterally and at the peripheral locations and it was improved in one (8%) patient.
Only one patient showed GGO and consolidations at the peripheral simultaneously. Pulmonary nodules were noted in the mediastinal region in 2 (15%) patients and in the subpleural region in one (8%) patient. Other changes observed were reticular changes in one (8%) patient, honeycomb changes at the bases of the lungs in another (8%) patient and apical lung fibrosis in only one (8%) patient. In this study 6 of the 11 patients with a low diffusion capacity were present with bilateral GGO in the chest CT, and 4 of them were previously treated for a rheumatological condition such as FM, RA and SLE. The patient with SLE showed bilateral upper lung fibrosis on her CT scan (Table 2).
6-Minute Walking Test (6MWT)
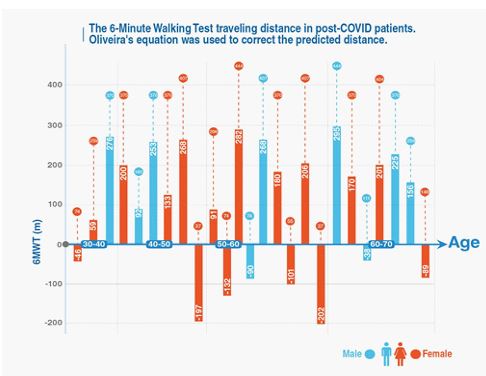
Twenty-five patients performed the 6MWT during the next three months after the onset of their symptoms. Five patients were unable to carry out the test. The 6MWT mean distance coverage by the 25 patients was 268.52 (SD,147.6) m (range, 37 to 444 m). The 6MWT mean distance walked for 15 women was 270 (SD,154.51) m (range, 37 to 444 m), and for 10 men the mean distance was 266 (SD,144.67) m (range, 74 to 444). Tachycardia (>120 bpm) was observed in 2 patients at the end of the study, one male who had barotrauma during his intubation and one female with obesity and hypothyroidism. Clinical significant desaturation (<90%) was observed in 4 patients associated with insufficient traveling distance, as a result of decreased FEV1 and DLCO with normal chest x-rays except in one patient.
We correlated the 6MWT distance (m) with the age and sex of each individual patient. Oliveira’s equation was used to correct the undependable variables (age, height, weight, sex and heart rate), and allow us to represent the range of distance that must be covered by each patient. We observed 8 survivors who presented with limited walking distance. Six of them were obese, 5 had at least one comorbidity such as acute kidney injury (AKI), rheumatoid arthritis (RA), end stage renal disease (ESRD), coronary artery disease (CAD) or congestive heart failure (CHF), 4 had low performance during the HGSC bilaterally and also they reported 5 domains at floor level on their SF-36 survey (PF, RP, GH, MH and EF), 3 of them had abnormal pulmonary function tests, 2 of them were afflicted by end stage renal disease and only one patient exhibited consolidations and pulmonary nodules on the imaging studies during her follow up evaluation (Figure 2).
Figure 2: The 6MWT Traveling Distance in Post-COVID Patients.
The 6 Minutes Walking Test was used to measure the traveling distance in our patients with post COVID symptoms. The Oliveira’s equation was used to correct the predicted distance. This figure has patients divided by sex and age group. Patients with negative values walked a shorter distance than the average person for that age group.
Musculoskeletal Evaluation (HGSC)
Sixteen patients completed the HGSC test during the first follow up evaluation between 3 to 6 weeks after their COVID outbreak. The mean HGSC for 10 women was 11 (SD,3.31) kgf for the right hand and 10 (SD,4.14) kgf for the left hand. The lowest outcome was obtained from a young lady with SLE, who had a mean of 5.4 kgf and 3.6 kgf at the right and left hand respectively. She became infected by shingles after COVID and showed pulmonary fibrosis at the CT of chest. The mean HGSC for 6 men was 28 (SD,16.99) kgf for the right hand and 26 (SD,16.12) kgf for the left hand. The lowest outcome was obtained from an adult man who was suffering with tendinitis and fatigue for one month. He had a mean of 10.4 kgf and 8.4 kgf at the right and left hand respectively. He also suffered from shingles after COVID and showed GGO and consolidations at the CT of chest.
Health Related Quality Of Life (HRQOL)
Fifteen patients were assessed through the SF-36 survey to evaluate the HRQoL after the outbreak. In our model of COVID-19 patients, HRQoL was affected by GH, RP, MH and EF, while PF, BP, SF and RE were less affected. The PF mean was 31 (SD,26.9), within a range 5 to 70; RP mean was 25 (SD,36.59), within a range 0 to 100; RE mean was 53 (SD,25.40), within a range 8 to 100; BP mean was 41 (SD,30.49), within a range 0 to 100; SF mean was 44 (SD,26,33), within a range 0 to 100; GH mean was 25 (SD,17.05), within a range 5 to 75; MH mean was 27 (SD,22.09), within a range 0 to 75; and the EF with a mean of 30 (SD,22.91), within a range 0 to 75.
Four patients were reported with the floor scores in the 8 domains of the HRQoL. The patient with the lowest score was an adult woman with previous asthma and morbid obesity, who was hospitalized for 2 weeks and showed consolidation at her chest X-rays and a decreased HGSC. The second low score was a young woman with SLE and status post shingles, who was not hospitalized by COVID pneumonia but was positive for around 2 months with an abnormal pulmonary function test (low FVC%, low FEV1% and low DLCO), decreased HGSC and a chest CT with pulmonary fibrosis. The third low score was reported for an adult woman with DM1, CAD and hypothyroidism, who was hospitalized with COVID pneumonia for 3 weeks and showed an abnormal pulmonary function test (low FVC%, low FEV1% and low DLCO), decreased HGSC and a chest CT with resolved GGO. The fourth patient developed AKI during his hospitalization for almost 2 months, though he was not intubated, and his pulmonary function tests were abnormal (low FVC%, low FEV1% and low DLCO), decreased HGSC and resolved GGO on his follow-up chest X-rays.
Discussion
To our knowledge, the medium and long-term consequences of post COVID-19 infection have been primarily analyzed in previous studies through phone or video call follow-ups, without appropriate face-to-face communication [22, 23, 24, 25, 26, 27]. In this study, patients underwent a complete inperson physical, respiratory, functional capacity and health related quality of life evaluation. We found that in those patients who have recovered from COVID-19, 100% reported persistence of symptoms, especially fatigue, body aches and sleep disturbance for more than 2 months. During their follow-ups, the examiners noted the presence of a general stress and negative psychological effect that COVID-19 infection had left on the quality of life of those patients. Of our population, 27 patients (80%) showed more than 6 MSP locations, more usually at the chest, upper back, lower back and limbs associated with fatigue and sleep disturbance for at least 3 months. These patients were educated and treated as FM patients during their follow-ups.
When we compared the data results with those obtained during the outbreak of SARS in 2003, we found multiple analogies that helped us to understand this pandemic better. During the first SARS pandemic, Hui et al [28], on his clinical study of 110 survivors follow-up for 6 months, found that many of them revealed multiple patchy GGO and interstitial thickening consistent with pulmonary fibrosis on their chest CT. Hui and colleagues assumed that those patients with SARS were undergoing an immune mediated alveolitis that led those patients to significant parenchymal fibrosis and lung function impairment. Subjects were unable to return to near normality until 6 to 12 months later, but the DLCO remained abnormal in up to 80% of patients after the first year of recovery. In effect the SARS-CoV-2 induced the alveolar cell death with the posterior accumulation of the apoptotic and necrotic cell detritus in the lumen of the alveolus and the subsequent liberation of macrophages-derivatives. This elicits a cytokine storm and finally promotes the hyaline membrane formation and the subsequent Acute Respiratory Distress Syndrome (ARDS) [29].
Xianoneng et al [30], described the pulmonary function in 110 hospital discharged patients with COVID-19 infection from mild illness to severe pneumonia. Abnormalities were noted in DLCO in 51 cases (47.2%), TLC in 27 (25%), FEV1 in 15 (13.6%), FVC in 10 (9.1%) and FEV1/FVC in 5 (4.5%). We performed pulmonary function in 16 patients and we got abnormalities in DLCO in 11 cases (69%), TLC in 8 (50%), FEV1 in 12 (75%), FVC in 10 (63%) and FEV1/FVC in 3 (19%). As a medium and long-term consequence of COVID-19 the impaired lung function associated with those imaging changes will be correlated with the fatigue, body aches, and sleep disturbance that our patients are showing during their follow-ups. These symptoms create the impression of being chronic fatigue syndrome/myalgic encephalomyelitis (CSF/ME) [31]. In 2015 the Institute of Medicine (IOM), now the National Academy of Medicine (NAM), established a new clinical diagnostic criteria for the CSF/ME, that requires the following three symptoms: “A substantial reduction or impairment in the ability to engage in pre-illness level of occupational, educational, social or personal activities, that persist for more of 6 months accompanied by fatigue”, “post-exertional malaise” and “unrefreshed sleep”. Additionally, either “cognitive impairment or orthostatic intolerance following other manifestations is required to be present [32].
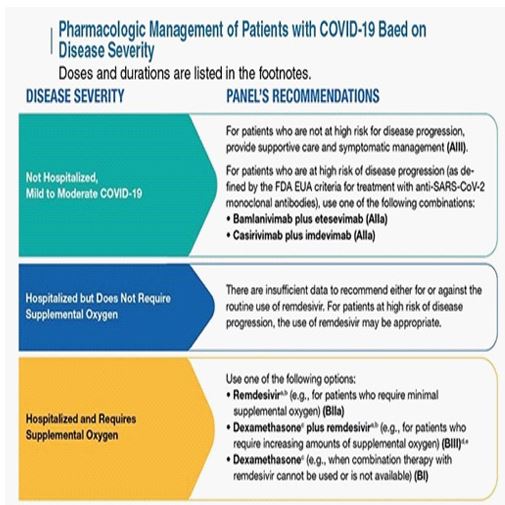
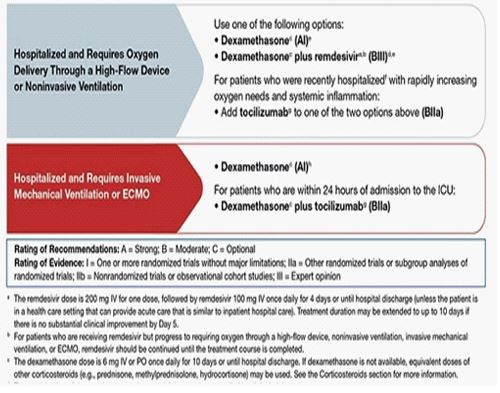
The IOM reported on its consensus “Beyond myalgic encephalomyelitis/chronic fatigue syndrome: Redefining an illness”, there is sufficient evidence to indicate that CFS/ME can follow infection with EBV and possibly other specific viral infections. For this reason, it is important how we follow our COVID-19 patients during the acute phase of the infection, such as Lerner et al, described in 2007 during the antiviral treatment of 54 patients to subset the CFS/ME symptoms [33]. It is mandatory that we enforce the pharmacological management of the National Institute of Health (NIH) based on the disease severity [34], to reduce the prevalence of the post-COVID acute syndrome (PACS) (Figure 3).
Figure 3: Pharmacologic Management of Patients with COVID-19 Based on Disease Severity.
Pharmacologic management of patients with COVID-19 based on disease severity developed by the National Institute of Health of US. Image downloaded from the National Health Institute www.covid19treatmentguidelines.nih.gov
In our practice we generated a questionnaire that helped us properly address our post-COVID evaluation, so we could better understand the most appropriate modality of treatment for each patient (Figure 4). The majority of patients complained about excessive fatigue and shortness of breath on exertion, and during their physical examination 29 (97%) exhibited tenderness over the trigger points. Many of those symptoms were associated with anxiety for not being able to return to their previous health status. Pulmonary rehabilitation (PR) was previously ordered only to one patient during his hospital discharge; however, after their follow-up evaluation 5 (17%) of those patients were included in the program of pulmonary rehabilitation at the Rahway Hospital. In review of pulmonary rehabilitation in COVID-19 patients, Md Abu Bakar and colleagues [35], found evidence that early participation in PR enhances exercise capacity, alleviates fatigue and strengthens the respiratory muscles in patients with various forms of respiratory disorders. The PR also reduces depression and anxiety and it improves the HRQoL.
Figure 4: Post-COVID-19 Evaluation Questionnaire.
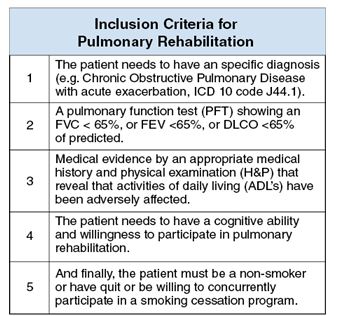
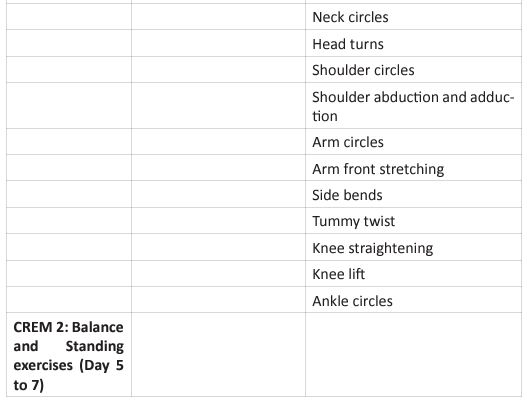
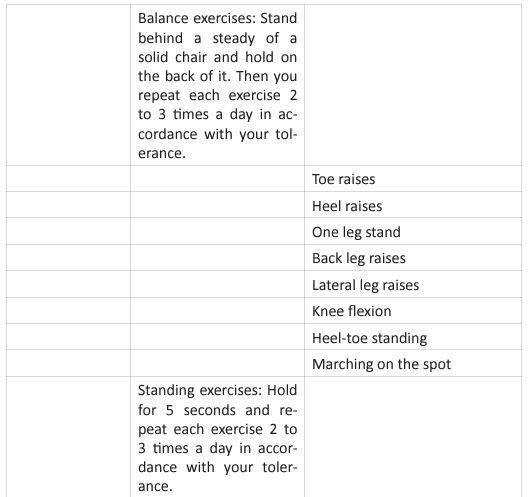
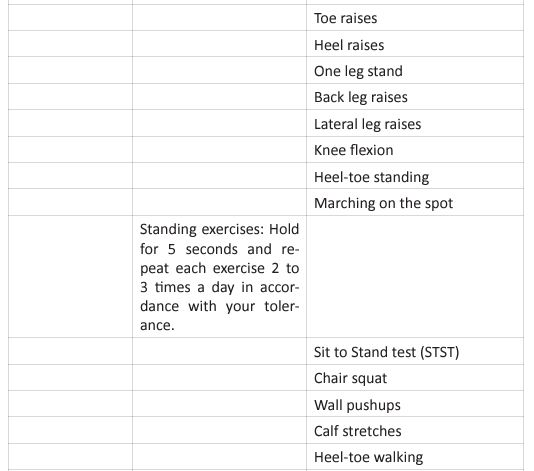
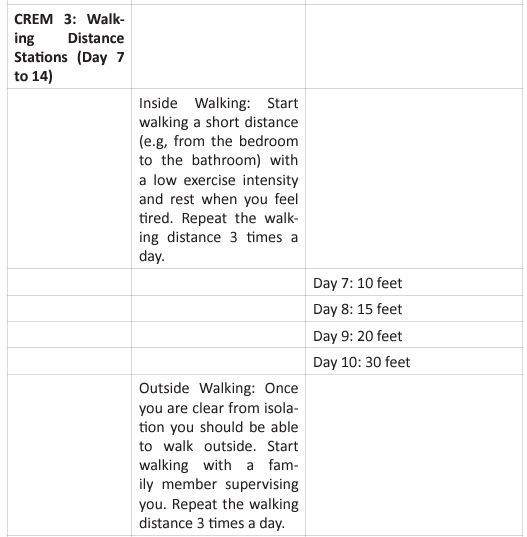
This questionnaire identifies the severity of the dysfunction generated by the disease in each patient. It also helps coordinate an appropriate medical and therapeutic plan. The inclusion criteria for PR were affected during the COVID-19 pandemic, due to the patients isolation, and their limitation to access to in-person rehabilitation. Around the world, for patients in isolation PR was conducted by educational videos, instruction manuals, or remote consultation (Table 3).
For critical inpatients, there should be no early PR interventions. Severe COVID-19 cases develop functional motor deficits impacting weaning from mechanical ventilation, long term outcomes and hospital mortality. Rehabilitation protocol includes active exercises performed at bedside, balance training in statics and dynamics, programs to prevent fall and lowintensity exercises of limb and trunk muscles. In the US, The PACER project (Post-Acute COVID-19 Exercise and Rehabilitation project) is a free series of modules led by APTA (Academic of Cardiovascular & Pulmonary Physical Therapy), and presented from a panel of leading experts to prepare clinicians for the challenges generated during the PACS rehabilitation [36]. Once the patient is referred to PR, and after an adequate H&P, the therapist needs to set up the goals of each individual patient. Those goals are based on 4 basic principles: (Figure 5).
Figure 5: Pulmonary Rehabilitation Four Basic Principles.
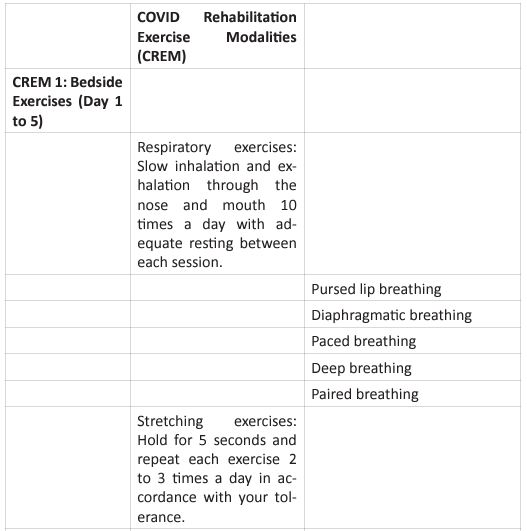
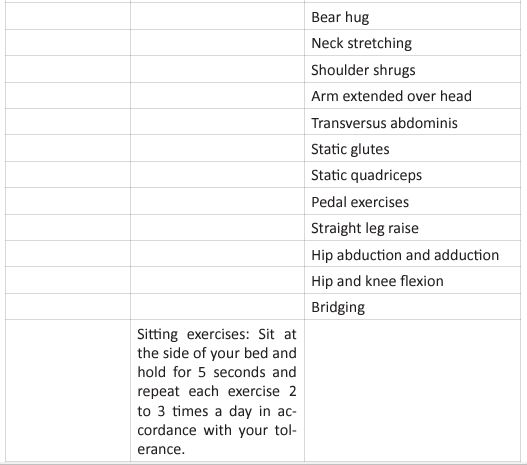
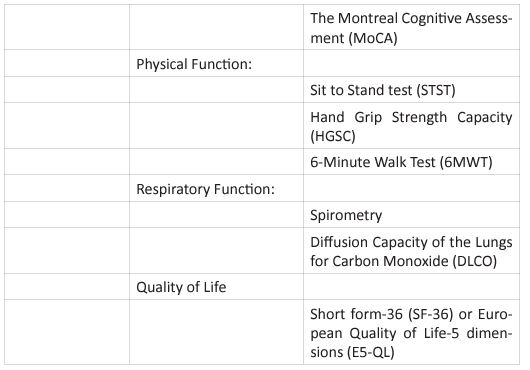
The graphic shows the four basic principles of pulmonary rehabilitation and how they are affected during the post-acute COVID syndrome. In this study, the COVID-19 survivors revealed a restrictive ventilation defect and impaired diffusion capacity, associated with GGO opacities on chest X-rays and CT of chest. Including in the most severe cases we were able to observe pulmonary fibrosis. Therefore, starting rehabilitation at an early stage of the COVID-19 infection is fundamental, because it decreases the possibility of those patients developing a severe disease. Hence, and following the dogmas formulated for Jian-Min Chen et al [37], during the application of the Eight-Segment rehabilitation exercise in people with COVID-19, we wrote the COVID Rehabilitation Exercise Modalities (CREM). The CREM protocol (Table 4) was designed to educate our patients during their post-COVID rehabilitation. It is indicated for patients who suffered a mild illness to a moderated COVID-19 pneumonia, and for those patients who are experiencing PACS. Patients must be free of fever/dyspnea/tachypnea/embolism and cardiac arrhythmias, and need to have adequate control of their blood pressure (<140/90) and oxygen saturation (>90%). Each patient needs to be educated about the use of the incentive spirometer and the pulse oximeter to be able to evaluate his/ her performance. Additionally, they must carry a daily logbook and contact our office in case of evidence of any oxygen desaturation less than 90% [38, 39]. Every patient should be evaluated by a physician before returning to work [40, 41]. At the moment of the follow-up visit, it is very important to have a negative rRT-PCR. There are a series of physical and therapeutic outcome measurements that include the following parts: the functional capacity questionnaire (Figure 6), the physical function evaluation (STST, HGSC
6MWT), the respiratory function evaluation (PFT’s, chest Xrays or CT chest), and the Health Related Quality of Life (SF36). In this study, we observed that many patients were unable to return to work due to their limitation to carry out their physical and therapeutic evaluations. At 6 months after COVID-19 onset, 18 patients (60%) were still experiencing at least one symptom, more frequently fatigue on exertion, dyspnea, multiple body aches and/or sleep disturbance. At the end of the study 4 (13%) patients were unable to return to work and needed to apply for a permanent disability [42, 43]. Post-acute COVID syndrome patients before returning to their previous jobs, must have a functional capacity assessment with a suitable tool. There are several limitations to this study. First, a small number of patients were included in the study, and from those only
Figure 6: Functional Capacity Questions.
16 to 25 (53 to 83%) survivors completed all the assessments over the 6 months. The reason for the default rate was the physical limitation due to their fatigue and shortness of breath during exertion. Second, 20 (67%) patients of our population had medical comorbidities that impacted their capacity to perform the assessments. Third, the most critical patients that were hospitalized for more than 6 months, were not reflected in this study.
Conclusion
This study has shown a significant reduction in the functional capacity and HRQoL of patients after COVID-19 infection. All of our patients showed fatigue, dyspnea, multiple body aches and sleep disturbance at the first month of follow-up. Almost half of the COVID-19 survivors showed a restrictive lung disease associated with an impaired DLCO, and GGO changes on the chest X-rays. After the 6 months of the study, 60% of the patients still complained about their lingering COVID symptoms. These chronic post-COVID symptoms were similar to those found in patients with chronic fatigue syndrome/fibromyalgia. We recommended our patient to initiate an early rehabilitation program called CREM, to mitigate the PACS.
References
- Polastri, Massimiliano & Nava, Stefano & Clini, Enrico & Vitacca, Michele & Gosselink, Rik. (2020). COVID-19 and pulmonary rehabilitation: Preparing for phase three. European Respiratory Journal. 55. 20018;22. [DOI: 10.1183/13993003.018222020].
- Knight, Matthew & Greenhalgh, Trisha. Management of post-acute covid-19 in primary care. BMJ (online). 2020. 370. 10.1136/bmj.m3026.
- Quartuccio L, Semerano L, Benucci M, Boissier MC, De Vita S. Pistes urgentes dans le traitement de l’infection par le COVID-19 : cibler l’inflammation en aval pour prévenir un syndrome catastrophique. Rev Rhum Ed Fr. 2020 May;87(3):146149. French. [PMID: 32355445; PMCID: PMC7181970 DOI: 10.1016/j.rhum.2020.03.009] [Epub 2020 Apr 24].
- Rowaiye, Adekunle & Onuh, Olukemi & Oli, Angus & Okpalefe, Okiemute & Oni, Solomon & Nwankwo, Ezinne. (2020). The pandemic COVID-19: a tale of viremia, cellular oxidation and immune dysfunction. Pan African Medical Journal. 36. 10.11604/pamj.2020.36.188.23476.
- Li, Bo & Yang, Jing & Zhao, Faming & Zhi, Lili & Wang, Xiqian & Liu, Lin & Bi, Zhaohui & Zhao, Yunhe. (2020). Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clinical Research in Cardiology. 109. 10.1007/s00392020-01626-9.
- Monteleone G, Sarzi-Puttini PC, Ardizzone S. Preventing COVID-19-induced pneumonia with anticytokine therapy. Lancet Rheumatol. 2020 May;2(5):e255-e256. [PMID: 32368737; PMCID: PMC7193140 DOI: 10.1016/S2665-9913(20)30092-8] [Epub 2020 Apr 6].
- Lippi, Giuseppe & Wong, Johnny & Henry, Brandon. Hypertension and its severity or mortality in Coronavirus Disease (COVID-19): a pooled analysis. Polskie archiwum medycyny wewnȩtrznej. 2019. 130. 10.20452/pamw.15272.
- Moldofsky, Harvey & Patcai, John. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC neurology. 2020. 11. 37. 10.1186/1471-2377-11-37
- Torgrimson-ojerio, Britta & Ross, Rebecca & Dieckmann, Nathan & Avery, Stephanie & Bennett, Robert & Dupree Jones, Kim & Guarino, Anthony & Wood, Lisa. Preliminary evidence of a blunted anti-inflammatory response to exhaustive exercise in fibromyalgia. Journal of Neuroimmunology. 2014. 277. 10.1016/j.jneuroim.2014.10.003.
- Steffen E. L, Paz da Silva J. E, Pillat M. M. Cytokines Involved in COVID-19 and Chronic Inflammatory Diseases: A Perspective from Fibromyalgia. Ann Clin Immunol Microbiol. 2020;2(1):1013.
- Lau HM, Lee EW, Wong CN, Ng GY, Jones AY, Hui DS. The impact of severe acute respiratory syndrome on the physical profile and quality of life. Arch Phys Med Rehabil. 2005 Jun;86(6):1134-40. [PMID: 15954051; PMCID: PMC7094462 DOI: 10.1016/j.apmr.2004.09.025].
- Johnny W.M. Chan and Chun Kong Ng, “ Severe Acute Respiratory Syndrome (SARS): A Brief Review With Exploration of the Outcomes, Prognostic Factors and Sequelae”, Current Respiratory Medicine Reviews. 2005;1:85. https://doi. org/10.2174/1573398052953659.
- Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The longterm impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010 Apr;15(3):543-50. [PMID: 20337995; PMCID: PMC7192220 DOI: 10.1111/j.1440-1843.2010.01720.x] [Epub 2010 Mar 19].
- Arnold LM, Bennett RM, Crofford LJ, Dean LE, Clauw DJ, Goldenberg DL, Fitzcharles MA, Paiva ES, Staud R, Sarzi-Puttini P, Buskila D, Macfarlane GJ. AAPT Diagnostic Criteria for Fibromyalgia. J Pain. 2019 Jun;20(6):611-628. [PMID: 30453109 DOI: 10.1016/j.jpain.2018.10.008] [Epub 2018 Nov 16]
- Rousan LA, Elobeid E, Karrar M, Khader Y. Chest x-ray findings and temporal lung changes in patients with COVID-19 pneumonia. BMC Pulm Med. 2020 Sep 15;20(1):245. [PMID: 32933519; PMCID: PMC7491017 DOI: 10.1186/s12890-02001286-5].
- Güneyli, Serkan & Atçeken, Zeynep & Doğan, Hakan & Altınmakas, Emre & Atasoy, Cetin. Radiological approach to COVID-19 pneumonia with an emphasis on chest CT. Diagnostic and interventional radiology (Ankara, Turkey). 2020. 26. 10.5152/dir.2020.20260.
- Liu, Dehan & Zhang, Wanshu & Pan, Feng & Li, Lin & Yang, Lian & Wang, Jiazheng & Zheng, et al. The Pulmonary Sequalae in Discharged Patients With COVID-19: A Short-term Observational Study. 10.21203/rs.3.rs-21985/v3.
- ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002 Jul 1;166(1):111-7. http://dx.doi. org/10.1164/ajrccm.167.9.950
- Oliveira, Maria João & Marçôa, Raquel & Moutinho, J. & Oliveira, P. & Ladeira, Ines & Lima, Roger & Guimaraes, Miguel. Reference equations for the 6-minute walk distance in healthy Portuguese subjects 18–70 years old. Pulmonology. 2018. 25. 10.1016/j.pulmoe.2018.04.003.
- Silva, Andréa & Garmatz, Eduardo & Goulart, Cássia & Carvalho, Lisiane & Cardoso, Dannuey & Paiva, Dulciane. HandGrip and Functional Capacity in Chronic Obstructive Pulmonary Disease patients. Physical Therapy in Movement. 2017. 30. 10.1590/1980-5918.030.003.ao08.
- OrthoToolKit SF-36. https://orthotoolkit.com/Sf-36 Accessed March 1, 2021.
- Xiong, Qiutang & Xu, Ming & Li, Jiao & Liu, Yinghui & Zhang, Jixiang & Xu, Yu & Dong, Weiguo. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2020. 27. 10.1016/j.cmi.2020.09.023.
- Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, Walshaw C, Kemp S, Corrado J, Singh R, Collins T, O’Connor RJ, Sivan M. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol. 2021 Feb;93(2):1013-1022. [PMID: 32729939 DOI: 10.1002/jmv.26368] [Epub 2020 Aug 17].
- Ontario Agency for Health Protection and Promotion (Public Health Ontario). Long-Term Sequelae and COVID-19 – What We Know So Far [Internet]. Toronto, ON: Queen’s Printer for Ontario; 2020 [cited 2012 Apr 12]. Available from: https:// www.publichealthontario.ca/-/media/documents/ncov/ covid-wwksf/2020/07/what-we-know-covid-19-long-termsequelae.pdf
- Living evidence - post acute sequelae of COVID-19. Agency for Clinical Innovation. https://aci.health.nsw.gov.au/covid-19/critical-intelligence-unit/post-acute-sequelae.
- Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020 Aug 11;324(6):603605. [PMID: 32644129; PMCID: PMC7349096 DOI: 10.1001/ jama.2020.12603].
- Huang, Chaolin & Huang, Lixue & Wang, Yeming & Li, Xia & Ren, Lili & Gu, Xiaoying & Kang, et al. Articles 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2021;397. 10.1016/S01406736(20)32656-8.
- Hui, D & Joynt, Gavin & Wong, Kin Tak & Gomersall, C & Li, Thomas & Antonio, G & Ko, Fanny & Chan, M & Chan, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005; 60. 401-9. 10.1136/ thx.2004.030205.
- Panigrahy, Dipak & Gilligan, Molly & Huang, Sui & Gartung, Allison & Cortes Puch, Irene & Sime, et al. Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19?. Cancer and Metastasis Reviews. 2020; 39. 10.1007/s10555-020-09889-4.
- Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, Lei C, Chen R, Zhong N, Li S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020 Jun 18;55(6):2001217. [PMID: 32381497; PMCID: PMC7236826 DOI: 10.1183/13993003.01217-2020].
- Marshall, Michael. The lasting misery of coronavirus longhaulers. Nature. 2020;585. 339-341. 10.1038/d41586-02002598-6.
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an illness. Washington (DC): National Academies Press (US); 2015 Feb 10. PMID: 25695122.
- Lerner, A.M., Beqaj, S.H., Deeter, R.G., Fitzgerald, J.T. Valacyclovir treatment in Epstein-Barr virus subset chronic fatigue syndrome: thirty-six months follow up. in vivo, 2017;21(5):707713.
- COVID-19 Treatment Guidelines. National Institutes of Health. Accessed: March 5, 2021. https://www.covid19treatmentguidelines.nih.gov/
- Siddiq, Md Abu & Rasker, Johannes. Pulmonary Rehabilitation in COVID-19 patients: A scoping review of current practice and its application during the pandemic. 2020;66:480-494. 10.5606/tftrd.2020.6889.
- Academy of Cardiovascular and Pulmonary Physical Therapy. Post-Acute Covid-19 Exercise & Rehabilitation (PACER) Project. Accessed: November 2,2020. https://www.aptacvp. org/pacer-project
- Chen, Jian-Min & Wang, Zhi-Yong & Chen, Yang-Jia & Ni, Jun. The Application of Eight-Segment Pulmonary Rehabilitation Exercise in People With Coronavirus Disease 2019. Frontiers in Physiology. 2020. 11. 646. 10.3389/fphys.2020.00646.
- World Health Organization Regional Office for Europe. Support for Rehabilitation Self-Management after COVID19-Related Illness. Available from: https://www.who.int/ publications/m/item/support-for-rehabilitation-self-management-after-covid-19-related-illness
- Physical Medicine & Rehabilitation (PM&R) Service, Washington DC Veterans Affairs Medical Center. Home Rehabilitation Program for COVID-19 Patients. Available from: https:// www.va.gov/covidtraining/docs/General_COVID_course_ files/Home_Rehabilitation_Program_for_COVID_19_Patients. pdf
- Torres-Castro R, Solis-Navarro L, Sitjà-Rabert M, Vilaró J. Functional Limitations Post-COVID-19: A Comprehensive Assessment Strategy. Arch Bronconeumol. 2021 Jan;57 Suppl 1:7-8. [PMID: 32943250; PMCID: PMC7455248 DOI: 10.1016/j. arbres.2020.07.025] [Epub 2020 Aug 28].
- Arnold DT, Hamilton FW, Milne A, Morley AJ, Viner J, Attwood M, Noel A, Gunning S, et al. Barratt SL. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2020 Dec 3;76(4):399-401. [PMID: 33273026; PMCID: PMC7716340 DOI: 10.1136/thoraxjnl-2020-216086] [Epub ahead of print].
- Podell R, Dimmock ME, Comerford BB. Documenting disability in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Work. 2020;66(2):339-352. [PMID: 32568153 DOI: 10.3233/WOR-203178.
- Tenforde, Mark & Kim, Sara & Lindsell, Christopher & Rose, Erica & Shapiro, Nathan & Files, Daniel & Gibbs,et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network — United States, March–June 2020. MMWR. Morbidity and Mortality Weekly Report. 2020; 69. 10.15585/mmwr.mm6930e1.