Case Report - Volume 2 - Issue 4
Guidance for planning, designing and running a HPV-based primary cervical cancer screening clinic in sub-Saharan Africa
Karolin Tatrai1*; Bruno Kenfack2; Jessica Sormani1,4; Pierre Vassilakos1,3; Patrick Petignat1
1Gynaecology Division, Department of Gynaecology and Obstetrics, University Hospitals of Geneva, Geneva, Switzerland.
2Department of Biomedical Sciences, University of Dschang, Dschang District Hospital, Dschang, Cameroon.
3Geneva Foundation for Medical Education and Research, Geneva, Switzerland.
4Geneva School of Health Sciences, HES-SO University of Applied Sciences and Arts Western Switzerland.
Received Date : June 06, 2022
Accepted Date : June 29, 2022
Published Date: July 20, 2022
Copyright:© Karolin Tatrai 2022
*Corresponding Author : Karolin Tatrai, Neumarktstrasse 62, 2503 Biel/Bienne, Switzerland. Tel: +41 76 446 03 79
Email:tatrai.karolin@gmail.com
DOI: Doi.org/10.55920/2771-019X/1196
Abstract
Cervical cancer is an important healthcare problem in sub-Saharan Africa and emphasis should be placed on implementing and strengthening capacities for screening and treating precancerous lesions. The World Health Organization’s recommendation for low- and medium-income countries to accelerate cervical cancer elimination is human papillomavirus-based primary screening followed directly by treatment or by triage with visual inspection with acetic acid then followed by treatment. To promote these approaches in a community health-care facility, a structurally and functionally dedicated cervical cancer screening and treatment clinic is required. Publications with practical details are rare in literature, imposing a challenge in such planning. In order to provide a toolkit and guidance for health professionals who plan to set up such a clinic, we herein report our experiences in the implementation and running of a cervical cancer screening and treatment clinic adapted to a district level.
Keywords: Cervical cancer; District hospital; Management; Prevention; Sub-Saharan Africa
Introduction
Cervical cancer (CC) is one of the leading causes of cancer death in women in sub-Saharan Africa (SSA) [1]. CC is caused by persistence of high-risk human papillomavirus (HPV) infection, which may lead to pre-cancer and then progress to cancer [2]. All of this process takes 10 to 20 years, making CC a preventable disease [3]. World Health Organization (WHO) estimates that 68’000 cases of CC are diagnosed annually in SSA, supporting the urgent need for implementation of preventive strategies to eliminate the CC burden [4].
The WHO’s strategy for low- and medium-income countries (LMIC) is HPV-primary screening followed either directly by treatment or by a triage test using visual inspection with acetic acid (VIA), and thereafter treatment for VIA-positive women [2]. Visual inspection-based triage aims to reduce overtreatment of HPV-positive women, focusing therapy on HPV and VIA-positive women, who have the highest risk of cervical intraepithelial neoplasia grade 2 or worse (CIN2+). Single-visit approach is recommended to reduce the number of consultations and to improve treatment compliance [5]. Although these approaches have been proven to be effective in a LMIC context, there are still significant challenges in implementing an organized screening program at national, regional land district levels [5]. The realization of a CC screening and treatment unit requires a structurally and functionally dedicated area and a well-organized process from diagnosis to treatment. It is also important that women who’s screening is positive receive treatment on the same day, in a single visit, reducing the risk of loss to follow-up [2].
Currently, there is still a lack of published experience concerning the operational aspect of this approach. The objective of this clinical paper is to identify the different problems and aspects of setting up a CC screening unit in SSA and to provide solutions for each of these issues. In the framework of a cooperation established in 1980 between the University of Yaounde, the Ministry of Public Health of Cameroon and the University of Geneva, a research project entitled “Comprehensive Cervical Cancer Prevention and Better Women Health” was initiated. The aim was to develop a CC prevention program tailored to the district level realities and needs. The strategy is to deliver CC screening and treatment outside of acute hospitals and closer to rural communities. This partnership helped to gain experience in screening site planning and organization, screening equipment, health worker training and supervision, protocol development, program monitoring and budgeting. This allowed us to replicate the structure in different places, ensuring an efficient delivery of care.
Clinical Problem
Even though strategies for CC screening and treatment of lesions in LMIC countries, such as proposed by the WHO (Figure 1) are well established, the problem lies often in the realization of such screening programs. In the following chapters, we will address problems that arise on different levels of the implementation of a CC screening unit, and propose solutions, with the knowledge gained through our multiple experiences in Cameroon.
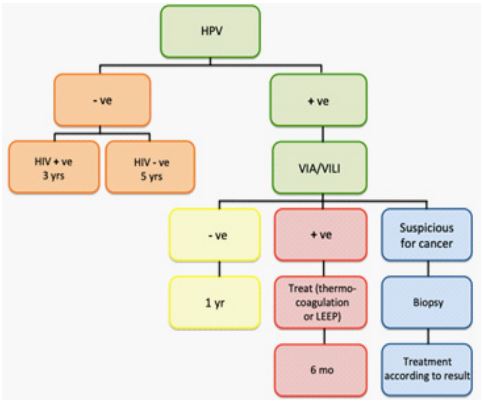
Figure 1: Screen and treat algorithm, adopted from WHO REF-PP0 [9].
General considerations
The model for a CC screening and treatment clinic reported here is a model adapted to local community hospitals aiming to deliver health care in rural areas. Designing and realizing a CC screening clinic in a peripheral area requires preparation, anticipation and planning. The approach reported here corresponds to the WHO screen and treat strategy (Figure 1).
The first step in designing a screening room is a needs analysis in the area where the screening should take place. This requires an assessment of the incidence of the disease in the region and calculation of target population at risk. To justify the infrastructure and workforce dedicated to this task, a critical number of daily 15 to 25 patients is required, assuming a routine screening day of approximately 6-8 working hours. Approximately 20% of the women will be HPV-positive and will need to undergo an additional exam (VIA triage) and eventually receive treatment. Assuming a 5-day working week, there will be 75 to 100 screening tests per week and 3500 to 5000 annually. In a rural area with a population of 200’000 inhabitants, assuming that 10% of the population are women aged between 30 and 49 years old [6], a total of 20’000 women would be eligible for screening. To screen 50 % of the target population, 10’000 screening tests will have to be performed over a period of 2-3 years.
Sizing a cervical cancer screening clinic
Sizing the unit and the different spaces needed has to be organized according to the setting and the scale of the clinic. The minimal space required for a CC screening and treatment room should be at least 30m2 and the different spaces can be set up according to (Figure 2). The workflow is in one direction and crossover circulation should be avoided, as shown in (Figure 2).
Briefing space/Waiting room (minimal 10 m2) – Briefing space should be large enough to accommodate about 20 people, allowing women’s health education and discussion. It can be located outside, allowing interested bystanders, and eventually local leaders to assist, listen and learn about the benefits of the screening and treatment program, to be able to pass on the information to their families and friends. This room can also be turned into a women’s health education centre to increase the awareness and uptake. All that is needed are small aids such as educational videotapes, flyers, and brochures, about women’s health issues, thereby providing education regarding sexual and reproductive health.
Admission area (minimal 5 m2) – Conducting patients’ interviews, including signing of consent forms (pelvic exam, biopsy and treatment), require individual attention and a space respecting patients’ privacy. This area may also be used to complete a clinical database, including sociodemographic data, clinical and laboratory data, and to see patients for scheduled follow-up visits.
Cabinet (minimal 2 m2) – Self-HPV test needs a separate area where women can discretely undress, do the swab in privacy and easily obtain assistance if needed.
Laboratory space (minimal 5 m2) – This space contains the HPV machine and the material required to perform the HPV test. It should be a quiet space for the laboratory staff to process the HPV tests and proceed to result interpretation.
Examination room (minimal 5 m2) – Space for patient to undress as well as for 2 staff members to move around freely to perform VIA/VILI procedure. Reusable and disposable equipment as well as the fluids needed to perform a proper VIA/VILI examination have to be housed in a compartmentalized space in order to work and to keep them sterile and proper, and have them close at hand for the exam.
Patient itinerary and procedure
The total screening time is approximately 2.5 hours for HPV-negative and 3.5 hours for HPV-positive women. The duration of the screening is an important issue to consider in the briefing and requires the counselling of participants before registration. Hereafter, we report a step-by-step description from screening to diagnosis and treatment and how to optimize the process (Figure 2 and 3). Our experience support that women accept these different steps performed by health workers, even though they had never been screened nor had any awareness about CC beforehand.
Step 1: Briefing and counselling (60 minutes) – Briefing focuses on anatomy with visual support (visualizing and naming internal female genital organs), and different medical issues such as what is cancer, cancer prevention, HPV and HPV transmission. Briefing is an interactive process that should be conducted in a way to inspire confidence and try to obtain women’s participation. This is a crucial part of the screening, as it helps to promote patients’ health education with emphasis on patient comprehension. Counselling and specific information for HIV positive women should also be provided. Proactive health promotion plays an important role in a CC screening clinic, and also aims to increase women’s participation in CC screening programs [7].
Step 2: Registration and consent (10 minutes) – Women interested to participate in the CC screening program are invited to provide demographic information and sign the appropriate consent forms. This step allows to outline the process and inform participants about possible adverse effects of the procedure and treatment, as well as to obtain their consent to perform the services provided. This enables participants to make an informed choice about whether or not to accept the screening. Interview and counselling should take place in an environment respecting patients’ privacy.
Step 3: Self-HPV (10 minutes) - The patient gets handed over a Self-HPV test tube, with precise instructions on how to do the test on herself. The woman then proceeds into a cabin, where she can do the HPV swab in privacy. Once having taken the swab, she hands over the test tube to the laboratory assistant for analysis. She is then lead to a hand-wash station, and afterwards asked to await her test results in a waiting room.
Step 4: HPV analysis (60 minutes) - Laboratory assistant prepares the samples then proceeds to the analysis.
Step 5: HPV test result restitution (10 minutes) - Once results are available, patients are invited one by one to announce their result, guarding privacy and confidentiality. These women are also informed about the next recommended screening round (Figure 1). Transmission of negative results takes about 5 minutes per patient. For the women who have a positive HPV result, not only the result is announced, but the following steps re explained as well: VIA/VILI process, possibility of immediate treatment with thermo-ablation and follow-up frequency according to the findings.
Step 6: VIA/VILI triage (20 minutes) - Before performing the gynaecological exam, patients are asked to empty their bladder. Pelvic examination is performed, vulva is carefully inspected, then speculum is inserted and VIA/VILI procedure performed, in association with digital imaging of the native cervix, and of the cervix after acetic acid and Lugol application [8]. The examiner finally determines if VIA/VILI is positive or negative, and decides whether to treat or to refer to a specialized centre if the results are suspicious for cancer. VIA/VILI should not only be used as a triage strategy but also to exclude a cervical cancer before initiating any treatment.
Steps 7: Treatment (20 minutes) – For HPV positive women or HPV and VIA/VILI positive, thermo-coagulation treatment is proposed and performed immediately. The procedure with its possible side effects is once again explained, the adequate probe head is selected according to the diameter and the position of the lesion [9]. The machine is preheated at 100°C during 60 seconds, and then the probe is applied to the lesion for 60 seconds. If the lesion is not entirely treated, further application(s) are performed. At the end of the treatment the cervix is checked for bleeding, which is then controlled by compression or application of Monsel’s solution. Before being discharged, each patient receives instruction concerning alert signs requiring a control and their follow-up date. Each patient gets a handout summarizing the screen and treat day as well as their HPV and VIA/VILI results. A hotline number is available 24/7, the phone being always with one of the local staff members.
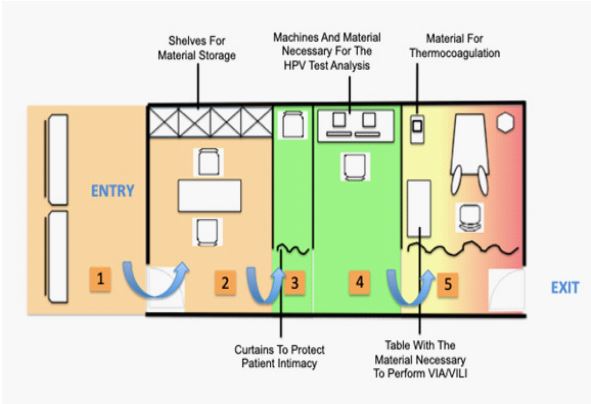
Figure 2: Sample architectural layout of a screening and treatment room for cervical cancer screening.
Note Figure 2: (1) Space for the briefing; (2) Desk to conduct patient interviews and counselling; (3) Cabin for HPV self-testing; (4) Laboratory space for HPV analysis; (5) Examination room for VIA/VILI triage and treatment with thermocoagulation.
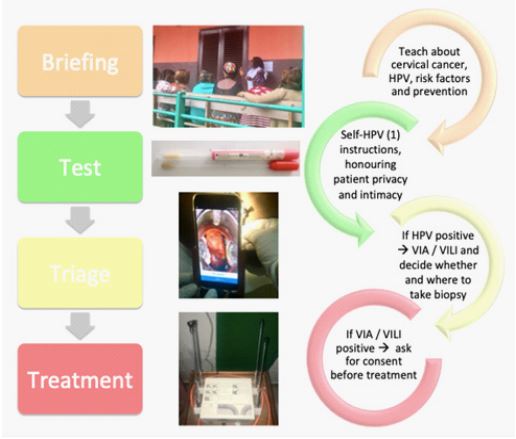
Figure 3: Workflow of women aged between 30-49 years old participating to cervical cancer screening.
Note Figure 3: (1) HPV self-sampling.
The Cervical Cancer Screening Team
Assuming an activity of 20 new participants per working day, the minimal staff needed to perform a test-triage-treatment based screening is at least 2 full-time health-care providers. To perform VIA/VILI at least 2 trained staff members are needed, one to perform the VIA/VILI exam and the second one to assist with the instruments, take digital photos, or provide assistance to the patient. Eventually a laboratory assistant may complete the team, having the responsibility to conduct the HPV testing. The clinics will also require a part time clinical leader, who acts as a clinical expert, responsible for the management and supervision of the clinical team. It can be a gynaecologist working in the hospital who may take on this role. On call support may be important, due to a small risk of bleeding, infection or hypotension after biopsies and treatment, therefore a support system i.e. by an experienced gynaecologist working close by, should be in place.
Training and supervision of primary care providers, as well as on-going quality control and quality assurance are essential [10]. Basic training takes a minimal of one week and should include theoretical and practical training courses. A training and operative procedure manual should be available for the staff. Training modules based on e-learning lectures, face-to-face courses, and practical training are provided by gynaecologists and midwives specialized in CC prevention. The content of the training should cover the natural history of HPV infection and prevention, VIA/VILI and treatment options. Staff supervision may include, (i) weekly formative supervision and a self-assessment logbook (1 hour), (ii) bi-monthly case review and training (2-3 hours) and, (iii) monthly evaluation of clinical practice (one day supervision by senior staff).
Equipment and infrastructure
Electricity supply – Many of the settings at peripheral levels have constraints with electricity supply in terms of both continuity and voltage fluctuations. Uninterrupted power supply is an essential requirement for successful work and this issue should be considered early in the process, as many devices depend on electricity (i.e. thermoablation machine, computers, lighting). Technical difficulties encountered due to frequent and sudden electrical cuts or high voltage variations may damage the equipment. Power failures don’t only cause for time and financial losses (i.e. HPV cartridge loss, electronic damage), but may pose an important issue for patients as well (i.e. treatment interruption). Frequent power shortages may delay patient management and even lead to loss of follow up, as they have to come back to complete the visit. An efficient backup system is generally based on a backup battery with an integrated power regulator to bridge power failures and voltage variation. Usually local specialists have the best expertise to install appropriate material and assume the maintenance of a power back-up solution.
Climatic influence and theft – Climate in LMIC can be extremely hot, dry or humid and this should be considered to adopt the screening room to these conditions. Planning for fans, a waterproof roof, dry spaces to store material, as well as protection to avoid dust exposure are mandatory. Also make sure that the screening facility, especially where material is stored, can be secured day and night, to prevent theft.
Material requirements – Examination room may be minimally equipped with the following essentials: gynaecological examination chair, material for a gynaecological examination, a chair for the examiner, curtains to separate spaces, a lamp for lighting, the equipment for the VIA/VILI, material for thermo-ablation (Figure 4). Concerning bureau material, protocols, pens, stickers for correct labelling, and folders to store the material are required.
Hygiene – To maintain an appropriate hygiene and prevent infection, disinfection fluids for hand sanitizing, surfaces and instruments are required. For the patients’ there needs to be a hand washing station, with soap and/or disinfectant, just before and after having performed Self-HPV test. After VIA/VILI and/or treatment of each patient, the space should be thoroughly cleaned as well as the equipment, completed with decontamination according to protocols. Make sure that sterilization is possible.
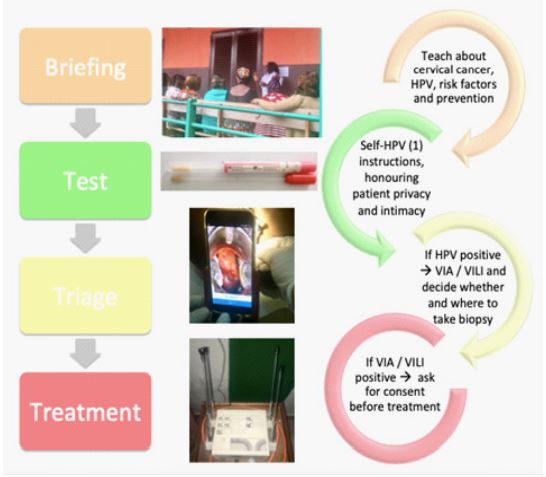
Figure 4: The examination room is minimally equipped with the essentials: gynaecological examination chair, a chair for the examiner, a lamp for lighting, the equipment for the VIA/VILI and D-VIA/D-VILI, and the essential materials for the thermo-ablation.
Note for Figure 4: (1) Gynaecological exam chair; (2) Mobile Lamp; (3) Smartphone to take the D-VIA /D-VILI photos; (4) Tripod for the Smartphone; (5) Thermo-ablation machine; (6) Different probe for thermo-ablation; (7) Power source to branch the electric devices.
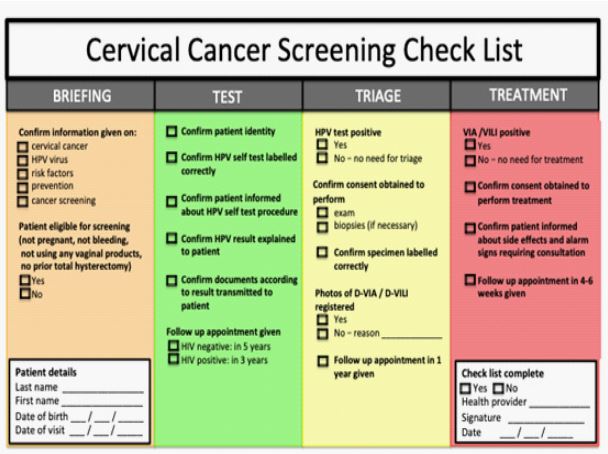
Figure 5: Checklist for quality control.
Hygiene – To maintain an appropriate hygiene and prevent infection, disinfection fluids for hand sanitizing, surfaces and instruments are required. For the patients’ there needs to be a hand washing station, with soap and/or disinfectant, just before and after having performed Self-HPV test. After VIA/VILI and/or treatment of each patient, the space should be thoroughly cleaned as well as the equipment, completed with decontamination according to protocols. Make sure that sterilization is possible.
Quality control
Crucial point prior to successful implementation of a new screening approach is to ensure the quality of care and patient safety. The system should be organized in a way to assure a continuous improvement in terms of diagnosis and treatment. This includes monitoring of providers and continuous training according to the observed weaknesses and needs. We established a checklist in order to help providers document appropriately the different steps of care, which is reported in (Figure 5). Since patient misidentification is one of the major potential risks, we have emphasized primary responsibility of health-care workers to check the identity of patients and match the correct patients with the correct samples and treatment. This strategy aims to provide safer care with significantly fewer errors.
Conclusion
Prevention is key, and with the optimization of adequate resources, a CC screening and treatment clinic can be successfully implemented in rural SSA. With an increasing number of LMIC introducing CC screening methods on a national or pilot basis, it is critical to ensure an adequate design and planning for setting-up a CC screening and treatment clinic. The development of such a clinic may have a large effect on improving the delivery of CC preventive care and to create some direction for future screening programs on a district level. This clinical report aims to provide a practical toolkit and guidance for health professionals in the planning of cervical cancer screening and treatment clinics, adapted to a district care level.
Competing interests: The authors declare that they have no competing interests.
Authors’ contributions: Each of the authors contributed substantially to the design, drafting, revision and final approval of the article.
Karolin Tatrai is a gynaecologic resident, who spent 3 months on site at the cervical cancer screening site in Cameroun, and worked at the cervical cancer screening unit at the University Hospitals of Geneva, gathering information and ideas on how to optimize the screening unit, therefore being the main author of this article.
Bruno Kenfack is a gynaecology specialist, working on site at the cervical cancer screening unit in Cameroun. He was at the source at the beginning of the screening programme, and helped with his local experience to set up the screening unit.
Jessica Sormani is a trained midwife and research assistant at the University Hospitals of Geneva, participation in the organisation of all research related to the cervical cancer screening programme.
Pierre Vassilakos is a pathologist working at the Geneva Foundation for Medical Education and Research and formerly working for the University Hospitals of Geneva, who has participated for over 20 years on the implementation of cervical cancer screening programs in Sub-Saharan Africa.
Patrick Petignat is the head of the department of gynaecology at the University Hospitals in Geneva, who helped organise, and constantly supervises the cervical cancer screening programme set up in Cameroun, as well as the related research.
References
- Cancer Mortality in Africa: International Agency for Research in Cancer, GLOBOCAN. 2018.
- WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. 2013.
- Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017; 141: 664-670.
- WHO Africa: Cervical cancer common amongst African women. 2017.
- Anorlu RI. Cervical cancer: the sub-Saharan African perspective. Reproductive Health Matters. 2008.
- Population Pyramid Cameroon. https://images.populationpyramid.net. 2019. Accessed 09 November 2019.
- Musa J, Achenbach CJ, O'Dwyer LC, et al. Effect of cervical cancer education and provider recommendation for screening on screening rates: A systematic review and meta-analysis. PLoS One. 2017; 12(9).
- Catarino R, Vassilakos P, Scaringella S, et al. Smartphone use for cervical cancer screening in low-resource countries: a pilot study conducted in Madagascar. PLoS One. 2015, Jul 29; 10(7).
- Viviano M, Kenfack B, Catarino R, et al. Feasibility of thermoablation in a screen-and-treat approach for the treatment of cervical precancerous lesions in sub-Saharan Africa. BMC Womens Health. 2017; 17(1): 2.
- REF-PP0: Monitoring national cervical cancer prevention and control programmes: quality control and quality assurance for visual inspection with acetic acid (VIA)-based programmes.

