Case Report - Volume 2 - Issue 4
Cushing’s disease secondary to a pancreatic Ewing Sarcoma in an 18-month-old child: a case report
Kate Conner1*; Rosemary Lane3; Matthew Drake2; Kiki Maoate5; Siobhan Cross; Martin de Bock1,4
1MBChb, Department of Paediatrics, University of Otago, Christchurch, New Zealand. University of Otago, Christchurch Campus 2 Riccarton Avenue, 8011 Christchurch, New Zealand.
2MBChb, Department of Pathology, Canterbury District Health Board, Christchurch, New Zealand.
3MBChb, Child Haematology and Oncology Centre, Canterbury District Health Board, Christchurch, New Zealand.
4MBChb, Department of Paediatrics, Canterbury District Health Board, Christchurch, New Zealand.
5MBChb, Department of Paediatric Surgery, Canterbury District Health Board, Christchurch, New Zealand.
Received Date : June 06, 2022
Accepted Date : July 01, 2022
Published Date: July 21, 2022
Copyright:© Kate Conner 2022
*Corresponding Author : Kate Conner, MBChb, Department of Paediatrics, University of Otago, Christchurch, New Zealand. University of Otago, Christchurch Campus 2 Riccarton Avenue, 8011 Christchurch,
New Zealand.
Email:kateliseconner@hotmail.com
DOI: Doi.org/10.55920/2771-019X/1197
Abstract
Background: Extraskeletal paediatric Ewing Sarcoma with resultant Cushing’s syndrome due to ectopic ACTH (adrenocorticotropic hormone) production is a very rare presentation with a poor prognostic outlook. This is the first pancreatic case ever reported.
Case presentation: An 18-month-old Caucasian female presented with rapid onset weight gain (2kg in two weeks), stridor and overnight apnoea, and irritability. She had a random paired plasma cortisol of 1115nml/L [170-550]) and ACTH of 6.5pmol/L [1-12 pmol/L] , and undetectable corticotrophin releasing hormone. An abdomen CT (computerised tomography) revealed a 64 x 69 x 80mm mass in the left upper quadrant which displaced the pancreatic tail and involvement of the splenic vasculature. After surgical resection, histopathology confirmed a diagnosis of Ewing Sarcoma. She received Children’s Oncology Group (COG) protocol AEWS1031, followed by consolidation chemotherapy, and gradually weaned hydrocortisone replacement. There were no signs of recurrence until a surveillance abdominal ultrasound scan 2 years after diagnosis revealed a 10mm porta hepatis lesion. Multiple further intra-abdominal lesions developed, and were unresponsive to 4 cycles of high dose ifosfamide. She was transferred to palliative care 31 months after her initial diagnosis with abdominal pain, hypertension and respiratory discomfort. Her plasma ACTH was raised at 18.6 pmol/L [1-2] with a random cortisol of 1232 nmol/L [170-500], and she redeveloped Cushingoid symptoms at 36 months post initial diagnosis. She died peacefully 37 months after her initial diagnosis.
Conclusions: There have only been six cases of ACTH producing Ewing Sarcoma reported, with this case being only the second extraskeletal one ever reported in literature, and the first that is pancreatic in origin. Regardless of treatment modality, no patients have survived for more than seven years after their initial diagnosis.
Keywords: Extraskeletal Ewings sarcoma; Cushing’s disease; paediatric; case report.
Abbreviations
ACTH: Adrenocorticotropic Hormone; CT: Computerised Tomography; USS: Ultrasound Scan; IV: Intravenous; ICU: Intensive Care Unit; MRI: Magnetic Resonance Imaging; COG: Children’s Oncology G1roup.
Background
Paediatric Ewing Sarcoma is a well reported condition with known treatment options and prognostic indicators. A very rare subset of this condition consists of ACTH producing tumours which cause a resultant Cushing’s syndrome. Existing literature describes five such cases ever reported, with only one extra-skeletal case. We present the presentation and clinical course of an ACTH producing extra-skeletal Ewing Sarcoma. The aim of this case presentation is to add a pancreatic case to the current literature and contribute to the limited knowledge of prognosis for these patients.
Case Presentation
An 18 month old Caucasian female presented with rapid onset weight gain (2kg in two weeks), stridor and overnight apnoea, and irritability. On examination she weighed 14kg (+ 2.45SD, 99th centile) with a height of 76.4cm (-1.48SD, 5th centile) with a BMI of 23.99(+4.68, >99thth centile). She was markedly hypertensive (100/50mmHG) (95th centile). She was cushingoid in appearance (central obesity, rounded face and dorsocervical fat pad) as seen in (Figure 1). Although she had a tanned complexion she had recently been on holiday in a Pacific Island country, and her tanning did not include non-sun-exposed areas. Hirsutism over the lower back was noted, but there was no pubic or axillary hair. The impression was that she had features consistent with Cushing’s disease and went forward for further investigation
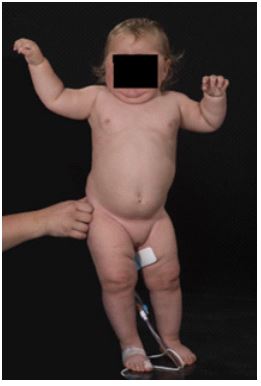
Figure 1: Cushingoid appearance at initial presentation at 18-months-old: moon facies, central adiposity, tanning limited to sun-exposed areas.
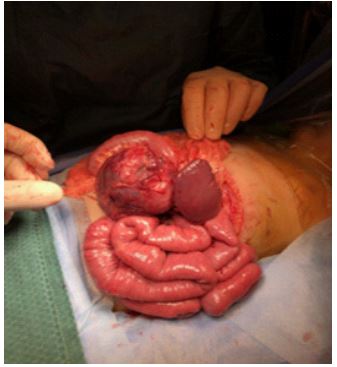
Figure 2: Perioperative image showing the tumour closely associated with splenic vasculature.
Laboratory results demonstrated paired random plasma cortisol of 1115nml/L [170-550]) and ACTH of 6.5pmol/L [1-12 pmol/L], and undetectable corticotrophin releasing hormone. A random urinary cortisol was 2711 nmol/L and a 24 hour collect was 996 nmol/day [<380 nmol/day]. Catecholamines were normal. Aldosterone was below detection limits, and renin was 49.2 mlU/L [5.3-99.1 mlU/L]. Glucose levels were in the normal range. A contrast CT of the abdomen showed a 64 x 69 x 80mm mass in the left upper quadrant with partial displacement of the pancreatic tail medially and splayed over the surface. The mass abutted the underside of the stomach without a clear fat plane. Although it closely abutted other organs in the area there was no suggestion of invasion. The splenic vasculature was intimately associated with the surface of the mass (Figure 2).
She went forward for surgical removal with a presumptive diagnosis of pancreatic neuroendocrine tumour with ectopic ACTH production. A transverse left upper quadrant incision was made. The mass was identified and dissection around the pancreas performed. Intra-operatively, as expected from pre-operative imaging, the mass was found to be closely associated with the splenic artery and splenic hilum requiring splenectomy to maximise the chance of adequate surgical margins. The tumour was removed en bloc with the spleen and tail of pancreas and the distal pancreatic tail oversewn. Histopathology is shown in (Figure 3), and confirmed Ewing sarcoma
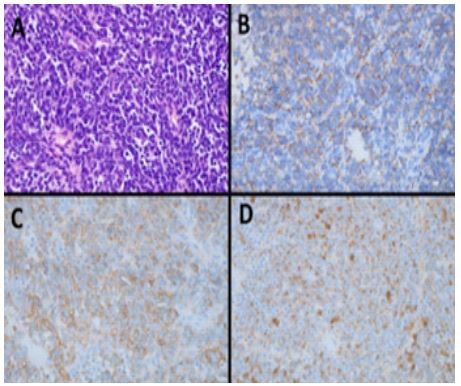
Figure 3: Histopathology.
Haematoxylin and eosin stained sections showed a malignant epithelioid tumour with small round blue cell morphology (A). Immunohistochemistry was positive for synaptophysin (B), CD99 (C) and cytokeratin AE1/3 (D).NXK2.2 immunohistochemistry was also positive, and subsequent fluorescent in situ hybridisation testing demonstrated an EWSR1 rearrangement, in keeping with Ewing sarcoma.
Post-operatively she recovered from the surgery well. Her blood pressure rapidly returned to normal. Endocrine management consisted of intra and post-operative hydrocortisone replacement at physiological levels. This was weaned over the following 5 weeks with subsequent normal adrenal axis testing. She received Children’s Oncology Group (COG) protocol AEWS1031- six cycles of alternating Vincristine/Doxorubicin/cyclophosphamide with Ifosfamide/etoposide. This was followed by consolidation chemotherapy of Vincristine, Doxorubicin, cyclophosphamide, Ifosfamide and etoposide.
After completing the six cycles she was discharged with a haemoglobin of 71 g/L [105-140], and returned the following day for a red blood cell transfusion. Twenty minutes into this she developed tachypnoea, tachycardia, fever and hypoxia, and was admitted to ICU (intensive care unit) for four days. She was treated with steroids, IV (intravenous) tazocin (for an episode of febrile neutropenia), high-dose co-trimoxazole and oxygen for a probable chemotherapy and transfusion-related acute lung injury of uncertain aetiology. Diffuse ground glass opacities were noted on chest CT. She subsequently developed pulmonary hypertension and right heart strain. She remained oxygen dependent for 14 months, and was gradually weaned. She was on prednisone for two years, and stopped sildenafil after 14 months. Chemotherapy was continued for another five months, during which time she had one ICU admission for an acute respiratory deterioration, but otherwise progressed well. Prior to starting prednisone, at 3 months post initial presentation, her morning basal cortisol levels were normal at 203 nmol/L [170-500]. They increased appropriately after Synacthen was administered to a peak of 1262 nmol/L [>400], demonstrating she had normal adrenocortical reserves.
Follow up radiology for the subsequent 14 months after completing chemotherapy showed no disease progression. She remained asymptomatic, but a surveillance abdominal USS (ultrasound scan) two years after her initial diagnosis found an indeterminate 10mm lesion in the porta hepatis region. A surveillance chest x-ray also found a possible new lung nodule, but this was later dismissed. A subsequent CT scan 25 months after her initial diagnosis found an ill-defined lesion in the portacaval region that extended into the porta hepatis, with hyper-attenuation of adjacent liver parenchyma. There were no other significant radiological findings. Abdomen MRI (magnetic resonance imaging) at 26 months found a further soft tissue nodule between the left adrenal and pancreatic tail, and involvement of multiple portal lymph nodes.
She received four cycles of high dose ifosfamide at 29 months post presentation, but further CT scans showed growth of all known disease sites, with the porta hepatis region at 14x19x27mm, and threatened occlusion of the duodenum, left portal and renal veins. No new disease sites were identified. No further chemotherapy was given. After stopping chemotherapy, her plasma ACTH was raised at 18.6 pmol/L [1-2] with a random cortisol of 1232 nmol/L [170-500] consistent with the recurrence ACTH secreting endocrine neoplasia.
She was transferred to palliative care at 31 months post initial presentation with abdominal pain, hypertension (138/72) despite ongoing antihypertensives, and increased respiratory discomfort due to hepatomegaly and decreased lung volume. At 36 months she developed Cushingoid features again with rapid weight gain (Figure 4), hyperpigmentation on extensor surfaces of joints, irritability, headaches and hypertension. Ketoconazole was started to try and alleviate her symptoms, however she deteriorated further and died peacefully 37 months after her initial diagnosis.
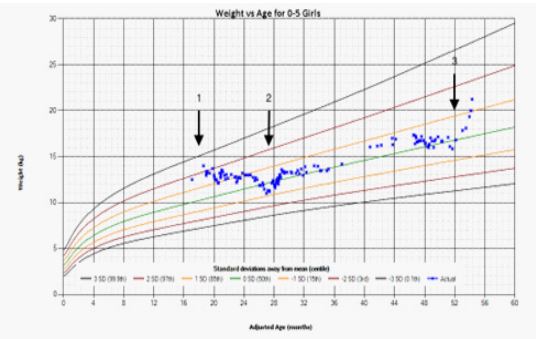
Figure 4: Growth Chart.
At presentation, Weight was above the 97th centile on diagnosis (arrow 1) and then decreased to 50th centile once in remission. Weight nadir was at completion of chemotherapy (arrow 2). Rapid weight gain coincided with disease relapse (arrow 3).
Discussion
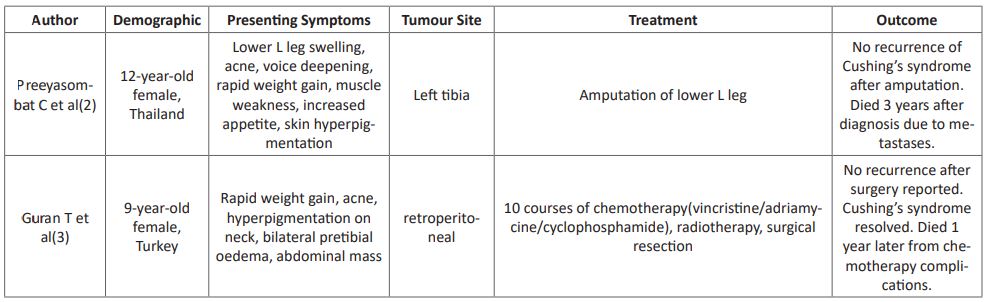
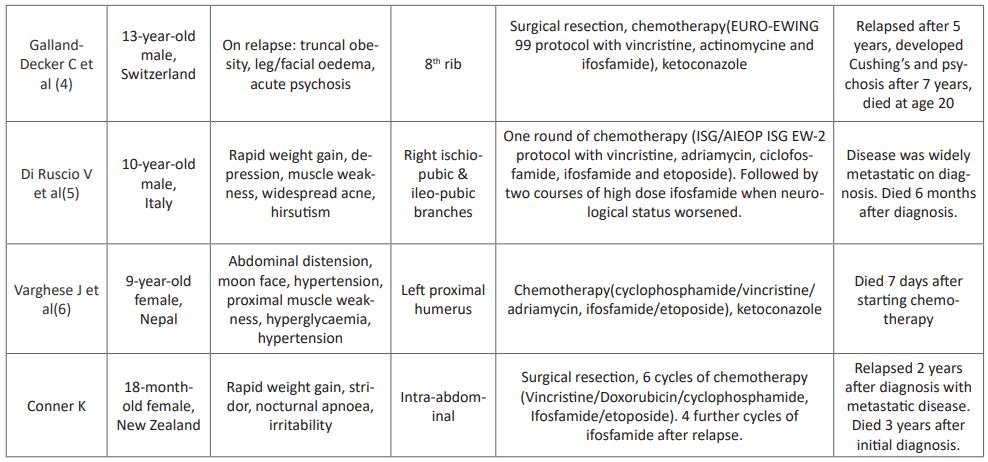
Here, we have reported the second case of extraskeletal Ewing sarcoma with Cushing’s syndrome in the literature. While extracranial and extra-adrenal tumours are well known to be able to cause Cushing’s syndrome it is rare association with Ewing sarcoma, with 5 cases reported over the last 27 years as summarised in (Table 1). The primary tumours varied in location, but only one other case was extraskeletal. All patients were diagnosed in childhood, and all died within 7 years of diagnosis, regardless of treatment modality or initial presentation.
For all extraskeletal Ewing sarcoma, a 5 year survival rate of 61% and disease free survival rate of 54% has been reported based on a small retrospective study [1]. It was not possible to determine if any of these were ACTH producing. We followed the COG surveillance guidelines in regards to Ewing sarcoma for this patient despite the unusual presentation with additional regular monitoring of hormonal levels, which is keeping with the other cases summarise in (Table 1). However, given that our case, and the others which have been reported all died within 7 years, infers that when Ewing sarcoma is ACTH producing the prognosis is much worse.
Table 1: Summary of published cases of ACTH producing Ewing Sarcoma.
Declarations
Ethics approval and consent to participate
Written consent for participation has been obtained from the patient’s parents. No ethics approval was required
Consent for publication
Written consent for publication and the use of images was obtained from the patient’s parents.
Availability of data and materials
Not applicable
Competing interests
The authors declare that they have no competing interests.
Funding
No funding was obtained for this manuscript
Authors’ contributions
KC and RL wrote the manuscript and reviewed the literature. MD performed the histopathology and wrote that section of the manuscript. KM was the principal surgeon. SC was the principal oncologist. MDB was the principal endocrinologist. All authors read and approved the final manuscript
Acknowledgements
Not applicable.
References
- Ahmad R, Mayol BR, Davis M, Rougraff BT. Extraskeletal Ewing's sarcoma. Cancer: Interdisciplinary International Journal of the American Cancer Society. 1999 ;85(3): 725-31.
- Preeyasombat C, Sirikulchayanonta V, Mahachokelertwattana P, Sriphrapradang A, Boonpucknavig S. Cushing's syndrome caused by Ewing's sarcoma secreting corticotropin releasing factor-like peptide. American Journal of Diseases of Children. 1992; 146(9): 1103-5.
- Guran T, Turan S, Ozkan B, Berrak S, Canpolat C, Dagli T, et al. Cushing's syndrome due to a non-adrenal ectopic adrenocorticotropin-secreting Ewing's sarcoma in a child. Journal of Pediatric Endocrinology and Metabolism. 2009; 22(4): 363-8.
- Galland-Decker C, Kraege V, Sartori C. Central nervous system manifestations due to iatrogenic adrenal insufficiency in a Ewing sarcoma patient. World journal of emergency medicine. 2019; 10(2): 119.
- Di Ruscio V, Del Baldo G, De Pasquale MD, De Vito R, Miele E, Colafati GS, et al. Ectopic ACTH Secretion in a Child With Metastatic Ewing's Sarcoma: A Case Report. Frontiers in oncology. 2020; 10: 574.
- Varghese J, Ayyagari M, Reddy SS, Mandapati NS, Subrahmanyam K. Rapidly Fatal Ectopic Adrenocorticotropic Hormone Syndrome in a 9-Year-Old Girl With Ewing Sarcoma. AACE Clinical Case Reports. 2021.

