Review Article - Volume 2 - Issue 4
Risk Factors, Diagnosis, Pathophysiology and Management of Deep Vein Thrombosis
Gudisa Bereda*
*Department of Pharmacy, Negelle Health Science College, Guji, Ethiopia.
Received Date : June 08, 2022
Accepted Date : July 04, 2022
Published Date: July 26, 2022
Copyright:© Gudisa Bereda 2022
*Corresponding Author : Gudisa Bereda, Department of Pharmacy, Negelle Health Science College, Guji, Ethiopia. Tel:+251913118492/+251919622717.
Email:gudisabareda95@ gmail.com
DOI: Doi.org/10.55920/2771-019X/1200
Abstract
Deep vein thrombosis occurs most often in the legs, but can form in the veins of the arms, and in the mesenteric and cerebral veins. Common risk factors of deep vein thrombosis includes age 40 years or older, being overweight, a personal or family history of blood clots, birth control pills, hormone replacement therapy, cancer, certain heart cases, stroke, respiratory failure, varicose veins, pregnancy, surgery including hip, knee, or stomach surgery, restricted mobility due to a long illness, injury, or surgery. Vascular injury that may result from major orthopedic surgery (e.g., knee and hip replacement), trauma (especially fractures of the pelvis, hip, or leg), or indwelling venous catheters. The D-dimer blood test measures degraded fibrinogen, which is raised in patients with a clot. The reference range varies and is set by the laboratory. This test is recommended in patients with a low or moderate clinical probability of deep vein thrombosis, as calculated by the Wells score. The two main types of anticoagulants are heparin and warfarin (coumadin) to keep a clot from growing or prevent new clots from forming. Warfarin interfere with hepatic synthesis of the vitamin K-dependent coagulation factors II, VII, IX and X. Warfarin, a vitamin K antagonist, is an effective and cheap oral anticoagulant.
Keywords: Deep vein thrombosis; Diagnosis; management; pathophysiology; risk factors.
Abbreviations
ACCP: American College of Chest Physicians; APTT: Activated Partial Thromboplastin Time; DOACs: Direct Oral Anticoagulants; DVT: Deep Vein Thrombosis; HIP: Heparin Induced Thrombocytopenia; HRT: Hormone Replacement Therapy; INR: International Normalized Ratio; LMWH: Low-Molecular-Weight Heparin; NICE: National Institute for Health and Care Excellence; PT: Prothrombin Time; PE: Pulmonary Embolism, TPA: Tissue Plasminogen Activator; UFH: Unfractionated Heparin; VKORC1: Vitamin K–Epoxide Reductase Complex 1
Introduction
The term thrombosis refers to the formation, from constituents of blood, of an abnormal mass within the vascular system of a living animal. When this process occurs within the deep veins, it is referred to as deep vein thrombosis (DVT). Deep vein thrombosis (DVT) commonly affects the lower limb, with clot formation beginning in a deep calf vein and propagating proximally [1, 2]. Deep vein thrombosis occurs most often in the legs, but can form in the veins of the arms, and in the mesenteric and cerebral veins [3]. DVT is common in lower extremities, pelvic venous system, renal venous system, upper extremity and right heart [4].
Risk factors
Risk of DVT associated with long-duration air travel is called economy class syndrome. It is 3% to 12% in a long-haul flight with stasis, hypoxia, and dehydration being pathophysiological changes that increase the risk. Genetic risk factors can be divided into strong, moderate, and weak factors. Strong factors are deficiencies of antithrombin, protein C and protein S. Moderately strong factors include factor V Leiden, prothrombin 20210A, non-O blood group, and fibrinogen 10034T. Weak genetic risk factors include fibrinogen, factor XIII and factor XI variants. Common risk factors of DVT includes age of 40 years or older, being overweight, a personal or family history of blood clots, birth control pills, hormone replacement therapy (HRT), cancer, certain heart cases, stroke, respiratory failure, varicose veins, pregnancy, surgery including hip, knee, or stomach surgery, restricted mobility due to a long illness, injury, or surgery [5-7].
Pathophysiology
Rudolph Virchow described three conditions that predispose to thrombus, the so-called Virchow’s triad. This triad includes endothelial injury, stasis or turbulence of blood flow, and blood hypercoagulability [8-10]. There are three primary components (virchov’s triad) rationale for pathogenesis of DVT such as venous stasis which slowed blood flow in the deep veins of the legs resulting from damage to venous valves, vessel obstruction, prolonged periods of immobility and increased blood viscosity; vascular injury that may result from major orthopedic surgery (e.g., knee and hip replacement), trauma (especially fractures of the pelvis, hip, or leg), or indwelling venous catheters and hypercoagulability which include malignancy; activated protein C resistance; deficiency of protein C, protein S, or antithrombin; factor VIII or XI excess; antiphospholipid antibodies; and other situations. Hypercoagulability is an important hallmark of inflammation. Pro-inflammatory cytokines are critically involved in abnormal lot formation and platelet hyperactivation and also play an important role in the downregulation of important physiological anticoagulant mechanisms. It has been determined that pro-inflammatory cytokines such as interleukin 6 (IL-6), IL-17A, and tumor necrosis factor reached increased levels in the majority of patients with severe outcomes [11, 12].
Diagnosis
The clinical presentation of DVT is often non-specific. Hence, accurate diagnosis requires sequential integration of clinical features, assessment of pre-test clinical probability, and confirmatory investigations that include D-dimer testing and imaging [13].
Symptoms and signs of deep vein thrombosis: Symptoms and signs of leg or pelvis DVT include leg pain, swelling, erythema and dilated superficial veins. Arm DVT has similar symptoms localized to the arm. Some DVTs are asymptomatic. Differential diagnoses for limb DVT include cellulitis, lymphoedema, chronic venous insufficiency, haematoma and, for leg DVT, ruptured Baker cyst [14].
D-dimer testing: The D-dimer blood test measures degraded fibrinogen, which is raised in patients with a clot. The reference range varies and is set by the laboratory. This test is recommended in patients with a low or moderate clinical probability of DVT, as calculated by the Wells score. Patients with a high clinical probability of DVT need not undergo the D-dimer test and should directly have ultrasonography. The test is easy to perform and readily available in secondary care. It has high sensitivity but is not very specific. Thus, a negative D-dimer can be used to exclude DVT in patients with a low clinical probability of DVT. However, it cannot confirm DVT, as D-dimer can be raised in other conditions including malignancy, infection, pregnancy, post-surgery, inflammation/trauma, disseminated intravascular coagulopathy, and renal impairment. An ultrasound is needed to confirm DVT [15, 16].
Table 1: Pretest probability assessment (Wells score).
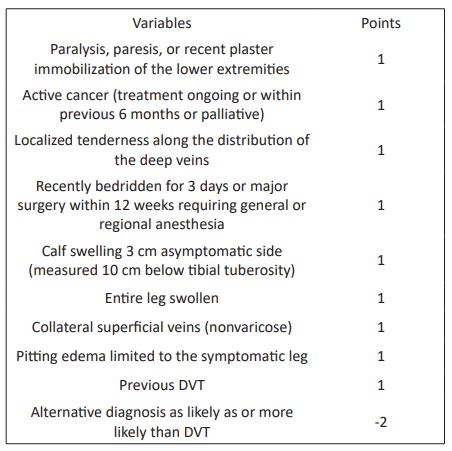
Clinical prediction rules
Although none of the symptoms or signs of DVT is diagnostic in isolation, it has been well established that a clinical prediction rule that takes into account signs, symptoms and risk factors can be accurately applied to categorize patients as having low, moderate or high probability of DVT. Alternatively, the same rule can be used to categorize cases as “DVT likely” or “DVT unlikely.” Patients who are found to be at low pretest probability can have DVT safely excluded on the basis of a single negative ultrasound result. Thus, serial ultrasound testing can be avoided in this subgroup of patients. The incorporation of plasma D-dimer testing into diagnostic algorithms can identify patients who do not require ultrasonography [17-20].
Treatment of deep vein thrombosis
The goal of therapy for DVT are to stop the clot from getting larger, reduce the chance of having another clot develop, prevent the extension of thrombus, acute PE, recurrence of thrombosis, development of late complications such as pulmonary hypertension and post-thrombotic syndromes, reduce the chance of the clot breaking off in your vein and moving to your lungs and to minimize adverse effects and cost of treatment [21, 22]. The two main types of anticoagulants are heparin and warfarin (coumadin) to keep a clot from growing or prevent new clots from forming [23]. The standard initial management of deep vein thrombosis has traditionally meant admission to hospital for continuous treatment with intravenous unfractionated heparin. Treatment then continued with a transition to long term use of oral anticoagulants (vitamin K antagonists). Recently a change has taken place, and low molecular weight heparins are being used. Guidelines prepared by the haemostasis and thrombosis task force recommend that patients receive heparin for at least four days and treatment should not be discontinued until the international normalized ratio has been in the therapeutic range for two consecutive days. According to these guidelines, a patient with a first episode of a proximal vein thrombosis should receive anticoagulants for six months, with a target international normalized ratio of 2.5 [24, 25].
Direct oral anticoagulants
Guidelines from National Institute for Health and Care Excellence (NICE) and American College of Chest Physicians (ACCP) recommends direct oral anticoagulants (DOACs) as first line treatment for DVT. DOACs include direct factor Xa inhibitors apixaban, rivaroxaban, and edoxaban, and a direct thrombin inhibitor, dabigatran (oral direct thrombin inhibitor) [26-28].
Warfarin
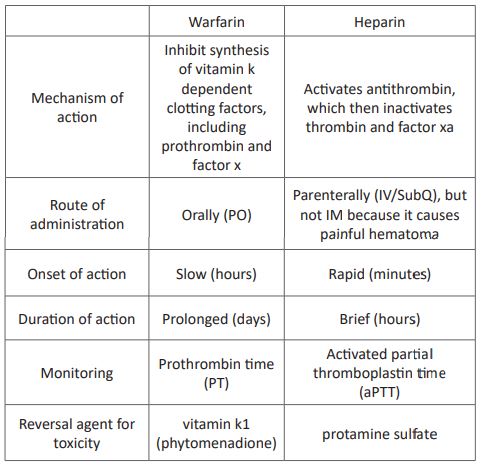
Warfarin interfere with hepatic synthesis of the vitamin K-dependent coagulation factors II, VII, IX and X or warfarin works by inhibiting vitamin K–epoxide reductase complex 1(VKORC1), the enzyme needed to convert vitamin K to the required active form. Warfarin reduces production of vitamin K–dependent clotting factors such as VII, IX, X, and prothrombin [29, 30]. Warfarin should begin concurrently with UFH or LMWH therapy. Warfarin, a vitamin K antagonist, is an effective and cheap oral anticoagulant [31]. Warfarin remains the drug of choice for long-term therapy to prevent clot formation once acute anticoagulation is achieved [32]. Warfarin should be administered, with a target INR of 2.0 to 3.0, for 3 months to patients with DVT following exposure to a transient risk factor (eg, surgery, trauma, immobility) and for at least 6 months to patients with unprovoked (or idiopathic) DVT [33]. Adverse drug reactions of warfarin are spontaneous haemorrhage, rashes, and teratogenesis from use during pregnancy [34]. Reversal agent of warfarin toxicity is vitamin k1 (phytomenadione). It bypasses inhibition of vitamin k epoxide reductase enzyme [35].
Heparins
Heparin inhibits coagulation by activating antithrombin III a protein that inactivates two major clotting factors: thrombin and factor Xa. Production of fibrin is reduced, and hence clotting is suppressed [36]. Heparin is initially given with warfarin and stopped after a minimum of 4 to 5 days, at which time the international normalized ratio (INR) should be within 2.0 to 3.0 (therapeutic range). This overlap with warfarin is essential because factors II, IX, X will not be affected until after 5 days, hence the intrinsic clotting pathway is intact. The initial prolongation of INR is mainly due to the effect of depression of factor VII which has a half-life of 5 to 7 hours [37, 38].
Low-molecular-weight heparin
Low molecular weight heparins are fragments of unfractionated heparin created by depolymerisation. The LMWHs increase the action of antithrombin III on factor Xa but not its action on thrombin, because the molecules are too small to bind to both enzyme and inhibitor, essential for inhibition of factor Xa but not for that of thrombin [39]. Several LMWH preparations, administered once or twice daily by subcutaneous injection, are currently approved for the initial treatment of DVT. Low-molecular-weight heparin preparations have several advantages over unfractionated heparin for treatment of DVT such as greater bioavailability, predictability and dose-dependent plasma level, less risk of bleeding, lower incidence of heparin-induced thrombocytopenia, lower risk of heparin-induced osteoporosis, no need for laboratory monitoring, can be safely administered in outpatient and duration of anticoagulant effect is longer, permitting once- or twice-daily administration [40-42].
Unfractionated heparin
Unfractionated heparin is a heterogeneous mixture of polysaccharide chains. The anticoagulant effect of UFH is mediated through a specific pentasaccharide sequence on the heparin molecule that binds to antithrombin, provoking a conformational change [43]. Outpatient treatment of DVT with twice-daily subcutaneous unfractionated heparin injections is efficacious and safe, based on level I evidence. Laboratory monitoring is required, however, with aPTT testing 6 hours after each daily morning dose. As with intravenous heparin therapy, subcutaneous heparin doses are adjusted to achieve a target aPTT of 1.5 to 2.0 times the control aPTT. One advantage of unfractionated heparin is that it is less expensive than LMWH [44]. Adverse drug reactions of heparin are bleeding, thrombocytopenia, HIP (heparin Induced Thrombocytopenia), osteoporosis and vertebral collapse; this is a rare complication described in young adult patients receiving heparin for longer than ten, skin necrosis at the site of subcutaneous injection after several days of treatment, alopecia, hypersensitivity reactions, including chills, fever, urticaria, bronchospasm and anaphylactoid reactions, occur rarely, hypoaldosteronism; heparin inhibits aldosterone biosynthesis [45, 46]. Reversal agent of heparin toxicity is protamine sulfate. Protamines are basic low-molecular-weight, positively charged proteins that have a high affinity for the negatively charged heparin molecules. The binding of protamine to heparin is immediate and results in the formation of an inert complex [47].
Table 2: The difference between warfarin and heparin
Thrombolytic (fibrinolytic) therapy
Fibrinolytic are proteolytic enzymes that enhance the conversion of plasminogen to plasmin, which subsequently degrades the fibrin matrix. It is given to remove thrombi that have already formed [48]. These are streptokinase, urokinase and alteplase.
Alteplase
is a thrombolytic drug, used to treat acute myocardial infarctions (heart attacks) and other severe conditions (ischemic stroke) caused by blood clotting by breaking up the blood clots that cause them. The recommended treatment dose of alteplase is 0.9 mg/kg (not to exceed 90 mg total treatment dose) infused over 60 minutes. 10% of the total treatment dose should be administered as an initial bolus over 1 minute. The remaining treatment dose should be infused intravenously over 60 minutes [49-51]. Catheter-directed thrombolysis involves the percutaneous insertion of a catheter and infusion of a thrombolytic typically recombinant tissue plasminogen activator (tPA) directly to the DVT [52].
Conclusion
Deep vein thrombosis (DVT) commonly affects the lower limb, with clot formation beginning in a deep calf vein and propagating proximally. Rudolph Virchow described three conditions that predispose to thrombus, the so-called Virchow’s triad. This triad includes endothelial injury, stasis or turbulence of blood flow, and blood hypercoagulability. The goal of therapy for DVT are to stop the clot from getting larger, reduce the chance of having another clot develop, prevent the extension of thrombus, acute PE, recurrence of thrombosis. Guidelines from National Institute for Health and Care Excellence (NICE) and American College of Chest Physicians (ACCP) recommend direct oral anticoagulants (DOACs) as first line treatment for DVT.
Acknowledgments
The author would be grateful to anonymous reviewers for the comments that increase the quality of this manuscript.
Data Sources: Sources searched include Google Scholar, Research Gate, PubMed, NCBI, NDSS, PMID, PMCID, Scopus database, Scielo and Cochrane database. Search terms included: risk factors, diagnosis, pathophysiology and management of deep vein thrombosis.
Funding: None.
Availability of data and materials: The datasets generated during the current study are available with correspondent author.
Competing interests: The author has no financial or proprietary interest in any of the material discussed in this article.
References
- Li Y et al. Development and validation of a prediction model to estimate risk of acute pulmonary embolism in deep vein thrombosis patients. Scientifc Reports, 2022; 12: 649.
- Mumoli N, Dentali F, Conte G, Colombo A, Capra R, Porta C, et al. Upper extremity deep vein thrombosis in COVID-19: Incidence and correlated risk factors in a cohort of non-ICU patients. PLoS ONE. 2022; 17(1): e0262522.
- Anton A, Campreciós G, Pérez-Campuzano V, Orts L, García-Pagán JC, Hernández-Gea V. The Pathophysiology of Portal Vein Thrombosis in Cirrhosis: Getting Deeper into Virchow’s Triad. J. Clin. Med. 2022; 11: 800.
- Permpikul C et al. Incidence of proximal deep vein thrombosis in medical critical care patients. Thrombosis Journal, 2022; 20: 5.
- Arabi YM, Al-Hameed F, Burns KEA, Mehta S, Alsolamy SJ, Alshahrani MS, et al. Adjunctive Intermittent Pneumatic Compression for Venous Thromboprophylaxis. N Engl J Med. 2019; 380(14): 1305-15.
- Zhang C, Zhang Z, Mi J, Wang X, Zou Y, Chen X, et al. The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine. 2019; 98(23): e15833.
- Koupenova M, Kehrel BE, Corkrey HA, Freedman JE. Thrombosis and platelets: an update. Eur Heart J. 2017; 38(11): 785-91.
- Galanaud JP, Monreal M, Kahn SR. Epidemiology of the post-thrombotic syndrome. Thromb Res 2018; 164: 100-109.
- Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J 2017; 49: 1601792.
- Wallace R, Anderson MA, See K, et al. Venous thromboembolism management practices and knowledge of guidelines: a survey of Australian haematologists and respiratory physicians. Intern Med J, 2017; 47: 436-446.
- Lim W, Le Gal G, Bates SM, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv 2018; 2: 3226-3256.
- Wells PS, Ihaddadene R, Reilly A, Forgie MA. Diagnosis of venous thromboembolism: 20 years of progress. Ann Intern Med 2018; 168: 131-140.
- Cohen AT. Extended thromboprophylaxis with betrixaban: a new standard for acute medically ill patients. Eur Heart J Suppl, 2018; 20: E1-E2.
- Weitz JI, Jaffer IH, Fredenburgh JC. Recent advances in the treatment of venous thromboembolism in the era of the direct oral anticoagulants. F1000Res 2017; 6: 985.
- Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. N Engl J Med 2017; 376: 1211-1222.
- Bikdeli B, Chatterjee S, Desai NR, et al. Inferior Vena cava filters to prevent pulmonary embolism: systematic review and meta-analysis. J Am Coll Cardiol 2017; 70: 1587-1597.
- Turner TE, Saeed MJ, Novak E, Brown DL. Association of inferior vena cava filter placement for venous thromboembolic disease and a contraindication to anticoagulation with 30-day mortality. JAMA Network Open 2018; 1: e180452.
- Connors JM. Thrombophilia testing and venous thrombosis. N Engl J Med 2017; 377: 1177-1187.
- Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med 2018; 378: 2010-2021.
- Foucar CE, Stein BL. JAK2 V617F mutation testing in patients presenting with hepatic and portal vein thrombosis. JAMA 2017; 317: 2228-2229.
- Feinberg J, Nielsen EE, Jakobsen JC. Thrombolysis for acute upper extremity deep vein thrombosis. Cochrane Database Syst Rev 2017; (12): CD012175.
- Cate-Hoek T, Amin EE, Bourman AC, et al. Individualised versus standard duration of elastic compression therapy for prevention of post-thrombotic syndrome (IDEAL DVT): a multicentre, randomised, single-blind, allocation-concealed, non-inferiority trial. Lancet Haematol 2018; 5: e25-e33.
- Amin EE, Bistervels IM, Meijer K, et al. Reduced incidence of vein occlusion and postthrombotic syndrome after immediate compression for deep vein thrombosis. Blood 2018; 132: 2298-2304.
- Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancerassociated venous thromboembolism. N Engl J Med 2018; 378: 615-624.
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 2018; 36: 2017-2023.
- Schulman S. How I treat recurrent venous thromboembolism in patients receiving anticoagulant therapy. Blood 2017; 129: 3285-3293.
- Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018; 132: 1365-1371.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Obstetrics. ACOG practice Bulletin No.196: Thronboembolism in pregnancy. Obstet Gynecol. 2018; 132: e1-17.
- Junichi H, Tomoaki I, Sekizawa A, Tanaka H, Nakamura M, Katsuragi S, et al. Recommendations for saving mothers’lives in japan: Report from the Maternal Death Exploratory Committee (2010–2014). J Obstet Gynaecol Res. 2016; 42: 1637-43.
- Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014; 35(3033–69): 3069a-a3079.
- Mark B, Casini A, Hoppe KK, Boehlen F, Righini M, Smith NL. Risks of venous thromboembolism after cesarean sections: a meta-analysis. Chest. 2016; 150: 572-96.
- Abe K, Kuklina EV, Hooper CW, Callaghan WM. Venous thromboembolism as a cause of severe maternal morbidity and mortality in the United States. Semin Perinatol. 2019; 43: 200-4.
- Teng F, Sun KY, Fu ZR. Tailored classification of portal vein thrombosis for liver transplantation: Focus on strategies for portal vein inflow reconstruction. World J. Gastroenterol. 2020; 26: 2691-2701.
- Bhangui P, Fernandes ESM, Di Benedetto F, Joo DJ, Nadalin S. Current management of portal vein thrombosis in liver transplantation. Int. J. Surg. 2020; 82: 122-127.
- Xian J, Tang Y, Shao H, Wang X, Zhang M, Xing T. Effect of portal vein thrombosis on the prognosis of patients with cirrhosis without a liver transplant: A systematic review and meta-analysis. Medicine 2021; 100: e25439.
- Fortea JI, Carrera IG, Puente A, Cuadrado A, Huelin P, Tato C, et al. Portal Thrombosis in Cirrhosis: Role of Thrombophilic Disorders. J. Clin. Med. 2020; 9: 2822.
- Nicoară-Farcău O, Soy G, Magaz M, Baiges A, Turon F, Garcia-Criado A, et al. New Insights into the Pathogenesis, Risk Factors, and Treatment of Portal Vein Thrombosis in Patients with Cirrhosis. Semin. Thromb. Hemost. 2020; 46: 673-681.
- Lu J, Zhang XP, Zhong BY, Lau WY, Madoff D, Davidson JC, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: Comparing east and west. Lancet Gastroenterol. Hepatol. 2019; 4: 721-730.
- Ren W, Zhang J, Chen Y, Wen M, Su Y, Zhao Y, Lu S, Wu J. Evaluation of Coagulation, Fibrinolysis and Endothelial Biomarkers in Cirrhotic Patients With or Without Portal Venous Thrombosis. Clin. Appl. Thromb. 2020; 26: 1076029620982666.
- Praktiknjo M, Trebicka J, Carnevale R, Pastori D, Queck A, Ettorre E, Violi F. Von Willebrand and Factor VIII Portosystemic Circulation Gradient in Cirrhosis: Implications for Portal Vein Thrombosis. Clin. Transl. Gastroenterol. 2020; 11: e00123.
- Molinari M, Fernandez-Carrillo C, Dai D, Dana J, Clemente-Sanchez A, Dharmayan S, et al. Portal vein thrombosis and renal dysfunction: A national comparative study of liver transplant recipients for NAFLD versus alcoholic cirrhosis. Transpl. Int. 2021; 34: 1105-1122.
- Basaranoglu, M. Increased prevalence of portal vein thrombosis in patients with nonalcoholic steatohepatitis-cirrhosis due to increased proinflammatory cytokines releasing from abdominal adipose tissue. Eur. J. Gastroenterol. Hepatol. 2020; 32: 458.
- Zanetto A, Senzolo M, Campello E, Bulato C, Gavasso S, Saggiorato G, et al. Determinants of increased thrombotic tendency in NASH cirrhosis: Not there yet! Transpl. Int. 2021; 34: 1325-1327.
- Bos S, Boom BVD, Kamphuisen P, Adelmeijer J, Blokzijl H, Schreuder T, Lisman T. Haemostatic Profiles are Similar across All Aetiologies of Cirrhosis. Thromb. Haemost. 2019;119: 246-253.
- Dong G, Huang XQ, Zhu YL, Ding H, Li F, Chen SY. Increased portal vein diameter is predictive of portal vein thrombosis development in patients with liver cirrhosis. Ann. Transl. Med. 2021; 9: 289.
- Gîrleanu I, Trifan A, Stanciu C, Sfarti C. Portal vein thrombosis in cirrhotic patients-It is always the small pieces that make the big picture. World J. Gastroenterol. 2018; 24: 4419.
- Lindberg-Larsen V, Ostrowski SR, Lindberg-Larsen M, Rovsing ML, Johansson PI, Kehlet H. The effect of pre-operative methylprednisolone on early endothelial damage after total knee arthroplasty: A randomised, double-blind, placebo-controlled trial. Anaesthesia 2017; 72: 1217-1224.
- Mei H, Jiang Y, Luo L, Huang R, Su L, Hou M, et al. Evaluation the combined diagnostic value of TAT, PIC, tPAIC, and sTM in disseminated intravascular coagulation: A multi-center prospective observational study. Thromb. Res. 2018; 173: 20-26.
- Driever EG, von Meijenfeldt FA, Adelmeijer J, de Haas RJ, Heuvel MCVD, Nagasami C, et al. Nonmalignant portal vein thrombi in patients with cirrhosis consist of intimal fibrosis with or without a fibrin-rich thrombus. Hepatology 2021.
- Lippi G, Favaloro EJ. Venous and Arterial Thromboses: Two Sides of the Same Coin? Semin. Thromb. Hemost. 2018; 44: 239-248.
- Diaz JA, Saha P, Cooley B, Palmer OR, Grover S, Mackman N, et al. Choosing a mouse model of venous thrombosis: A consensus assessment of utility and application. J. Thromb. Haemost. 2019; 17: 699-707.
- Bos S, Boom BVD, Kamphuisen P, Adelmeijer J, Blokzijl H, Schreuder T, Lisman T. Haemostatic Profiles are Similar across All Aetiologies of Cirrhosis. Thromb. Haemost. 2019; 119: 246-253.

