Research Article- Volume 2 - Issue 5
The Association between Preoperative Anti-Tuberculosis Variability with the Resolution of Spondylitis Tuberculosis in Indonesia
Singkat Dohar Apul Lumban Tobing1; Muhammad Triadi Wijaya1; Thariqah Salamah2; Kevin Jonathan Adhimulia3*; Holong Mangasah3; Budi Susanto3; Kanthi Soraca3
1Department of Orthopedic and Traumatology, University of Indonesia, Indonesia.
2Department of Radiology, University of Indonesia, Indonesia.
3Faculty of Medicine, University of Indonesia, Jl. Salemba Raya No.6, RW.5, Kenari, Kec. Senen, Kota Jakarta Pusat, Jakarta 10430,Indonesia.
Received Date : July 18, 2022
Accepted Date : Aug 18, 2022
Published Date: Sep 03, 2022
Copyright:© Kevin Jonathan Adhimulia 2022
*Corresponding Author : Kevin Jonathan Adhimulia, Jl. Salemba Raya No.6, RW.5, Kenari, Kec. Senen, Kota Jakarta Pusat, Jakarta 10430, Indonesia.Tel: (+62)85212701235
Email:kevinj.adhimulia@yahoo.com
DOI: Doi.org/10.55920/2771-019X/1233
Abstract
Indonesia. Surgical management is indicated persistent symptoms despite the initiation of pharmacological treatment. Despite several studies that described preoperative administration of anti-tuberculosis drugs, there was no conclusion about the recommended duration and regiment to reduce the risks of complications.
Aim: We aimed to observe the association between preoperative anti-tuberculosis drugs duration and regiment with the resolution of spondylitis tuberculosis in the Indonesian population.
Method: We conducted an observational retrospective cohort study in Cipto Mangunkusumo General Hospital, Jakarta, from January 2010 to December 2019. All patients with spondylitis tuberculosis who had undergone surgical intervention were included in this study. Subjects were collected using consecutive sampling through the medical record and Department of Radiology database. Analysis was conducted to compare the duration and regiment of preoperative anti-tuberculosis drugs with the time of fusion and postoperative complications.
Results: We obtained 31 eligible subjects with spondylitis tuberculosis with a history of surgical intervention. We found no association between the subject's characteristics with the time of fusion and postoperative complications of surgical intervention in spondylitis tuberculosis. Association between anti-tuberculosis drug regiment and duration also showed no statistically significant difference of time to fusion and postoperative complications in surgically treated spondylitis tuberculosis.
Conclusion: Variability of anti-tuberculosis drug duration and regiment does not associate with the time of fusion and postoperative complication in surgically treated spondylitis tuberculosis.
Keywords: Spondylitis tuberculosis; preoperative; antituberculosis drugs.
Introduction
Tuberculosis is still one of the most common infectious diseases in developing countries, including Indonesia. World Health Organization (WHO) reported a total of 10, 4 million cases of Tuberculosis globally, with 1,7 million died annually in 2016. Tuberculosis is also registered as the top ten cause of mortality in 2016 [2]. The Ministry of Health in Indonesia reported 320.000 cases of Tuberculosis in Indonesia [2]. Most of the morbidity and mortality are caused by the complications of Tuberculosis, including spondylitis tuberculosis. Spondylitis tuberculosis is quite common in Indonesia due to the high prevalence of pulmonary Tuberculosis. Previous reports showed a high prevalence of spondylitis tuberculosis in Indonesia due to the high prevalence of pulmonary Tuberculosis [3,4]. Report has shown that 20% of all pulmonary Tuberculosis spread to extrapulmonary organs, including osteoarticular Tuberculosis [5]. Half of the osteoarticular Tuberculosis is spondylitis tuberculosis [5].
In general, the management of spondylitis tuberculosis includes pharmacological therapy with anti-tuberculosis drugs and surgical management. The current anti-tuberculosis regiment issued by the WHO recommended the combination of Rifampicin, Isoniazid, Pyrazinamide, and Ethambutol (RHZE) as the first line of treatment with the duration of 9-12 months for spondylitis tuberculosis. The treatment includes an intensive phase with two months of combining all four anti-tuberculosis drugs followed by a continuation phase with 7-10 months of both Rifampicin and Isoniazid [1,2]. Surgical management is indicated in chronic pain, progressive deformity, and neurological deficit despite the initiation of pharmacological treatment [6,7].
Previous studies have reported that preoperative administration of anti-tuberculosis drugs is indicated to minimize the complication of surgical procedures. However, the duration and the regiment of preoperative anti-tuberculosis drugs varies among studies with the duration of 1-2 weeks, 2-4 weeks, 4-5 weeks, or more than 8-12 weeks before surgical management [8-13]. Furthermore, the regiment of anti-tuberculosis drugs administered in the previous study has not been appropriately described other than a study by Wang et al., who described intensive regiment [9]. Despite several studies that described preoperative administration of anti-tuberculosis drugs, there was no conclusion about the recommended duration and regiment to reduce the risks of complications. We aimed to observe the association between preoperative anti-tuberculosis drugs duration and regiment with the resolution of spondylitis tuberculosis in the Indonesian population.
Method
We conducted an observational retrospective cohort study that aimed to evaluate the association between anti-tuberculosis regiment variability and spondylitis tuberculosis's surgical outcome. Anti-tuberculosis variability includes duration and combination of drug usage. This study was conducted in Cipto Mangunkusumo General Hospital, Jakarta, from January 2010 to December 2019. All patients with spondylitis tuberculosis who had undergone surgical intervention were included in this study. Subjects with spondylitis infection other than Tuberculosis, osteoporosis, and other spinal disorders which not correlated with the nature of spondylitis tuberculosis were excluded from the study.
Subjects were collected using consecutive sampling. Data were collected through the medical record and Department of Radiology database. Data collected includes the subject's general characteristics, spinal segment, degree of deformity, neurological deficit, and the presence of multidrug resistance tuberculosis prior to infection, history of anti-tuberculosis drug, comorbidities, type of surgical intervention, and postoperative outcomes time of fusion and complications. Time of fusion was evaluated on radiological x-ray examination that showed radiological fusion. Analysis was conducted to compare the duration and regiment of preoperative anti-tuberculosis drugs with the time of fusion and postoperative complications. The association between the subject's characteristics with the time of fusion and postoperative complication will also be conducted. Ethical approval of this study has been granted by the Medical Ethical Committee Faculty of Medicine, University of Indonesia.
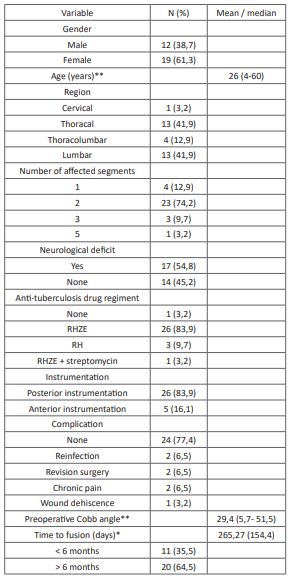
Table 1: Subject’s characteristics (N= 31).
*Normal distribution, reported in mean (standard deviation)
** Abnormal distribution, reported in median (min-max)
Results
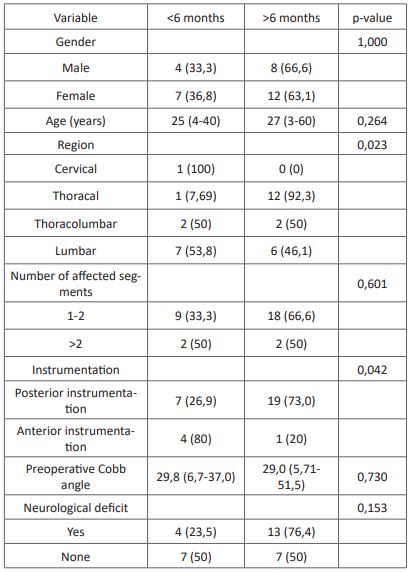
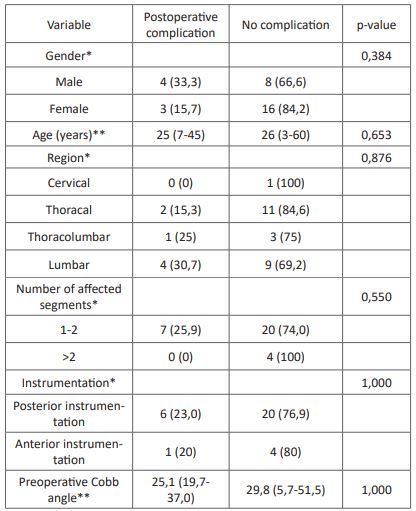
We obtained 31 eligible subjects with spondylitis tuberculosis with a history of surgical intervention. The subject's characteristics are presented in (Table 1). We found no association between the subject's characteristics with the time of fusion and postoperative complications of surgical intervention in spondylitis tuberculosis (Table 2). Association between anti-tuberculosis drug regiment and duration also showed no statistically significant difference of time to fusion and postoperative complications in surgically treated spondylitis tuberculosis (Table 3).
Table 2: Association between characteristics and time of fusion.
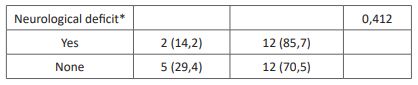
Table 3: Association between characteristics and complication.
* Analysis with Fisher exact test
** Analysis with Mann-Whitney test
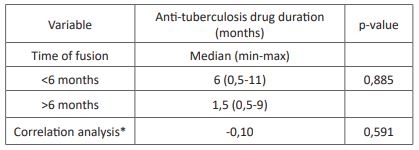
Table 4: Association between anti-tuberculosis drug duration and time of fusion.
*Correlation analysis using Spearman test, presented with a coefficient of correlation
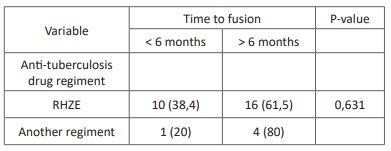
Table 5: Association between anti-tuberculosis drug duration and time of fusion.
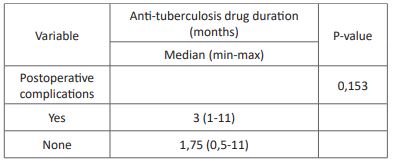
Table 6: Association between anti-tuberculosis drug duration and postoperative complications.
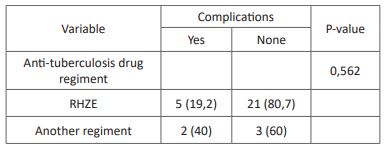
Table 7: Association between anti-tuberculosis drug duration and postoperative complications.
Discussion
The subject's characteristics of this study showed female predominance with a median age of 26 years old. The previous study has demonstrated gender predominance in spondylitis tuberculosis with various outcomes [14-17]. Furthermore, gender did not show any association with neither time of fusion nor postoperative complications. However, a higher proportion of postoperative complications occurred in the male gender, which is also shown in a previous study [18]. Predominance in the male gender could be associated with a difference in lifestyle and comorbidities that predispose to male complications [19]. The median age of the subject is also consistent with the previous study, especially in endemic countries [16,19]. We observed that the thoracal and lumbar region is the most common segment affected by spondylitis tuberculosis, which is consistent with the previous study [16,17,20,21]. This could be correlated with the hematogenous spread of Tuberculosis through the arterial arcade and subchondral region of each vertebra, which is more prevalent in the thoracal lumbar regions [22]. In this study, we found a significant association between the vertebral region and time of fusion with a faster time of fusion in the lumbar and thoracolumbar region compared with the thoracal region. Speedier time of fusion could be related to the higher vascularization of the lumbar region compared with other vertebrae.
Surgical intervention conducted in this study includes debridement, decompression, and instrumentation of the spine, including anterior and posterior instrumentation. Posterior instrumentation was performed in the majority of the subject (83,9%). The previous study has reported a lower complication rate with stronger spine stabilization than anterior instrumentation [23-25]. In this study, we found a significantly faster time of fusion in posterior instrumentation compared with anterior instrumentation despite no difference in postoperative complications.
The time of fusion in this study was evaluated radiologically with the median of 265 days or 8-9 months after surgical intervention with fusion less than six months in 35% of the subjects. The previous study has shown various results on time of fusion in spondylitis tuberculosis after surgical intervention. A study in Hong Kong reported that only 28% of the subjects had radiological union six months after surgical intervention, with the median union time between 6-12 months [26]. Another study in India showed even lower results, with only 8% of the subjects having radiological fusion before six months [27]. We found no significant association between anti-tuberculosis drug variability with the time of fusion in this study. However, we found a longer anti-tuberculosis drug duration (6 months) in the time to fusion <6 months group compared to > six months group (1,5 months). The results showed faster fusion time with a longer duration of anti-tuberculosis drug use in spondylitis tuberculosis. However, a previous study has stated that delay in surgical intervention due to anti-tuberculosis drug administration is not recommended due to lower neurological outcome [28].
The anti-tuberculosis regiment used in this study includes the recommended anti-tuberculosis drugs Rifampicin, Isoniazid, Pyrazinamide, and Ethambutol (RHZE), Rifampicin + Isoniazid (RH), RHZE + streptomycin, and no anti-tuberculosis use before surgical intervention. The standard drug regiment for spondylitis tuberculosis includes using RHZE for the intensive phase (first two months) followed by using RH for the continuation phase (7 months). In this study, we found a higher proportion of patients with the time of fusion <6 months in patients using RHZE (38%) compared with another regiment (20%) despite no statistical significance. Another anti-tuberculosis drug regimen could lead to multidrug resistance tuberculosis, leading to worse surgical outcomes and longer fusion time [29].
Postoperative complications including reinfection, revision surgery, chronic pain, and wound dehiscence were reported in 22,6% of the subjects in this study. The association of anti-tuberculosis regimens with postoperative complications showed no significant results despite a lower rate of postoperative complications in subjects with the RHZE regiment. A previous study by Journeau et al. showed similar results with no postoperative complication with the RHZE regiment for three months before surgical intervention [30]. Other studies by Colmenero et al. reported postoperative complications in 31% of all subjects with RHE or RHZ regimen for two months before surgical intervention [31]. Reinfection is the most prevalent postoperative complication, which is lower in subjects with RHZE regiment than in other regimens. The previous study has shown a lower rate of reinfection in patients treated with anti-tuberculosis drugs [30]. The association between anti-tuberculosis drug duration with postoperative complications showed no significant results. A previous study by Loenhout-Rooyackers et al. showed similar results with no difference in relapse between anti-tuberculosis drug use six months and over six months [32]. Furthermore, the outcome of postoperative outcome could be affected by preoperative conditions and the operator [30].
The limitation of this study includes the retrospective and consecutive nature of this study and limited variable that could be controlled. Thus, several surgically treated patients with spondylitis tuberculosis could not be included in this study due to loss to follow-up. Furthermore, the follow-up x-ray examination to conclude the time of fusion was not controlled, causing increased risks of bias. We also could not control several confounding factors such as anti-tuberculosis drugs adherence.
Conclusion
Variability of anti-tuberculosis drug duration and regiment does not associate with the time of fusion and postoperative complication in surgically treated spondylitis tuberculosis.
Conflict of Interests: Authors declares no conflict of interests.
References
- World Health Organization. Guidelines for treatment of drug-susceptible Tuberculosis and patient care: 2017 update. 2017.
- Kementrian Kesehatan Republik Indonesia. Pedoman Nasional Pelayanan Kedokteran: Tata Laksana Tuberkulosis. 4th ed. Kementrian Kesehatan RI; 2014.
- Singh G, Sunggoro A. Profil Pasien Tuberkulosis Berat dalam Perawatan Inap di Rumah Sakit Cipto Mangunkusumo, Jakarta. Indonesian Journal of Chest. 2015; 2(1): 16-9.
- Evayanti LG, Kalanjati VP, Machin A. A rare widespread tuberculous spondylitis extended from the T5-T10 levels – a case report. IOP Conf Ser: Mater Sci Eng. 2018 Dec 4; 434: 012323.
- Faried A, Hidayat I, Yudoyono F, Dahlan R, Arifin M. Spondylitis Tuberculosis in Neurosurgery Department Bandung Indonesia. JSM Neurosurgery and Spine. 2015 Aug 14; 3: 1-4.
- Rajasekaran S, Soundararajan DCR, Shetty AP, Kanna RM. Spinal Tuberculosis: Current Concepts. Global Spine Journal. 2018 Dec; 8(4): 96S-108S.
- Rasouli MR, Mirkoohi M, Vaccaro AR, Yarandi KK, Rahimi-Movaghar V. Spinal Tuberculosis: Diagnosis and Management. Asian Spine J. 2012; 6(4): 294.
- Dai L-Y, Jiang L-S, Wang Y-R, Jiang S-D. Chemotherapy in anterior instrumentation for spinal Tuberculosis: highlighting a 9-month three-drug regimen. World Neurosurg. 2010 May; 73(5): 560-4.
- Wang X, Pang X, Wu P, Luo C, Shen X. One-stage anterior debridement, bone grafting and posterior instrumentation vs. single posterior debridement, bone grafting, and instrumentation for the treatment of thoracic and lumbar spinal Tuberculosis. Eur Spine J. 2014 Apr; 23(4): 830-7.
- Ling T, Liu L, Yang X, Qiang Z, Hu X, An Y. Revision surgery for spinal tuberculosis with secondary deformity after treatment with debridement, instrumentation, and fusion. Eur Spine J. 2015 Mar; 24(3): 577-85.
- Huang Q-S, Zheng C, Hu Y, Yin X, Xu H, Zhang G, et al. One-stage surgical management for children with spinal Tuberculosis by anterior decompression and posterior instrumentation. Int Orthop. 2009 Oct; 33(5): 1385-90.
- Zhao J, Lian XF, Hou TS, Ma H, Chen ZM. Anterior debridement and bone grafting of spinal Tuberculosis with one-stage instrumentation anteriorly or posteriorly. Int Orthop. 2007 Dec; 31(6): 859-63.
- Li M, Du J, Meng H, Wang Z, Luo Z. One-stage surgical management for thoracic Tuberculosis by anterior debridement, decompression and autogenous rib grafts, and instrumentation. Spine J. 2011 Aug; 11(8): 726–33.
- PROCOPIE I, POPESCU EL, PLEȘEA RM, DOROBANȚU M, MUREȘAN RF, LUPAȘCU-URSULESCU CV, et al. Clinical-Morphological Aspects in Spinal Tuberculosis. Curr Health Sci J. 2018; 44(3): 250-60.
- Dharmalingam M. Tuberculosis of the spine-the Sabah experience. Epidemiology, treatment and results. Tuberculosis (Edinb). 2004; 84(1–2): 24-8.
- Godlwana L, Gounden P, Ngubo P, Nsibande T, Nyawo K, Puckree T. Incidence and profile of spinal tuberculosis in patients at the only public hospital admitting such patients in KwaZulu-Natal. Spinal Cord. 2008 May; 46(5): 372–4.
- Kim C-J, Song K-H, Jeon J-H, Park WB, Park SW, Kim H-B, et al. A Comparative Study of Pyogenic and Tuberculous Spondylodiscitis: Spine. 2010 Oct; 35(21): E1096–100.
- Wang B, Kong L, Zhu Z, Gao W, Guo H, Wang X, et al. Recurrent complex spinal Tuberculosis accompanied by sinus tract formation: causes of recurrence and clinical treatments. Sci Rep. 2018 Dec; 8(1): 6933.
- Alavi SM, Sharifi M. Tuberculous spondylitis: Risk factors and clinical/paraclinical aspects in the south west of Iran. Journal of Infection and Public Health. 2010 Dec 1; 3(4): 196–200.
- Trecarichi EM, Di Meco E, Mazzotta V, Fantoni M. Tuberculous spondylodiscitis: epidemiology, clinical features, treatment, and outcome. Eur Rev Med Pharmacol Sci. 2012 Apr; 16(2): 58–72.
- Öztürk AM, Yener C, Taşbakan Işikgöz M. CURRENT CONCEPTS ON SPINAL TUBERCULOSIS. jtss. 2020 Feb 21; 31(1): 60–3.
- Garg RK, Somvanshi DS. Spinal Tuberculosis: A review. The Journal of Spinal Cord Medicine. 2011 Sep; 34(5): 440–54.
- Yi Z, Song Q, Zhou J, Zhou Y. The efficacy of single posterior debridement, bone grafting and instrumentation for the treatment of thoracic spinal Tuberculosis. Sci Rep. 2021 Feb 11; 11(1): 3591.
- Cui X, Ma YZ, Chen X, Cai XJ, Li HW, Bai YB. Outcomes of different surgical procedures in the treatment of spinal Tuberculosis in adults. Med Princ Pract. 2013; 22(4): 346–50.
- Alam MdS, Phan K, Karim R, Jonayed SA, Munir HKMd, Chakraborty S, et al. Surgery for spinal Tuberculosis: a multi-center experience of 582 cases. J Spine Surg. 2015 Dec; 1(1): 65–71.
- Mak KC, Cheung KMC. Surgical treatment of acute TB spondylitis: indications and outcomes. Eur Spine J. 2013 Jun; 22(4): 603–11.
- Singh R, Magu N, Rohilla R. Clinicoradiologic Profile of Involvement and Healing in Tuberculosis of the Spine. Ann Med Health Sci Res. 2016; 6(5): 311–27.
- Agradi P, Hidajat NN, Ramdan A. Effect of Preoperative Anti Tuberculosis Drug Administration Duration on Tuberculous Spondylitis Surgical Treatment Outcomes. Jurnal Anestesi Perioperatif. 2020 Apr 12; 8(1): 9-16.
- Ma Y, Pang Y, Du J, Liu Y, Li L, Gao W. Clinical outcomes for multi- and extensively drug resistant tuberculosis patients with adjunctive resectional lung surgery in Beijing, China. J Thorac Dis. 2017 Mar; 9(3): 841–5.
- Loembe PM. Tuberculosis of the lower cervical spine (C3-C7) in adults: diagnostic and surgical aspects. Acta Neurochir (Wien). 1994; 131(1–2): 125-9.
- Colmenero JD, Jiménez-Mejías ME, Sánchez-Lora FJ, Reguera JM, Palomino-Nicás J, Martos F, et al. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. 1997 Dec; 56(12): 709–15.
- van Loenhout-Rooyackers JH, Verbeek ALM, Jutte PC. Chemotherapeutic treatment for spinal Tuberculosis. Int J Tuberc Lung Dis. 2002 Mar; 6(3): 259-65.