Case Report - Volume 2 - Issue 5
Recurrent Perianal Squamous Cell Carcinoma in a Retroviral Patient
Ussof Eskaandar Mohd Hussain1*; Jothinathan Muniandy; Fitzjerald Henry
Department of Surgery, Hospital Selayang, Selangor, Malaysia.
Received Date : July 21, 2022
Accepted Date : Aug 24, 2022
Published Date: Sep 07, 2022
Copyright:© Jing Wang 2022
*Corresponding Author : Ussof Eskaandar Mohd Hussain, Department of Surgery, Hospital Selayang, Selangor, Malaysia.
Email:ussof_69@yahoo.com
DOI: Doi.org/10.55920/2771-019X/1236
Abstract
Background: Anal squamous cell carcinoma is a cancer strongly associated among the immunocompromised and those infected with Human Papilloma Virus. We present a case of locally advanced recurrent anal cancer in a retroviral disease patient and the treatment planning involved in managing this patient.
Case presentation: In this report, we present a 51 years old male with retroviral disease, presenting with recurrent perianal squamous cell carcinoma. We decided to excise the lesion as he has no systemic metastasis combined with plastic and reconstructive team.
Conclusion: Surgical resection can be offered in patient with recurrent anal squamous cell cancer with no systemic metastasis.
Keywords: Anal squamous cell carcinoma; Recurrent anal cancer; Retroviral disease.
Introduction
Anal cancer only constitutes 4% of all the cancers of lower gastrointestinal tract. The main etiology is infection with human papilloma virus (HPV). Patients infected with Human Immunodeficiency Virus (HIV) is an independent risk factor in developing anal cancer. The increasing trend of patients with squamous cell carcinoma (SCC) of anus is a reflection of high prevalence of HPV in the population. This is a case of a retroviral positive patient developing local recurrence of SCC 4 years following local resection of anal growth and chemoradiotherapy. Patient underwent Laparoscopic assisted abdominoperineal resection with wide excision of perineum and V-Y advancement flap construction.
Case report
We describe a case involving a 51 years old male retroviral positive patient on HAART therapy presenting with an anal growth back in 2017. Local excision of the growth was taken on initial suspicion of anal warts. His histopathological (HPE) was reported as anal squamous cell carcinoma with involved margin. He underwent chemotherapy combined with high dose radiotherapy. Following that his surveillance Contrast Enhanced Computed Tomography (CECT), colonoscopy and regular per rectal assessment showed no evidence of recurrence.
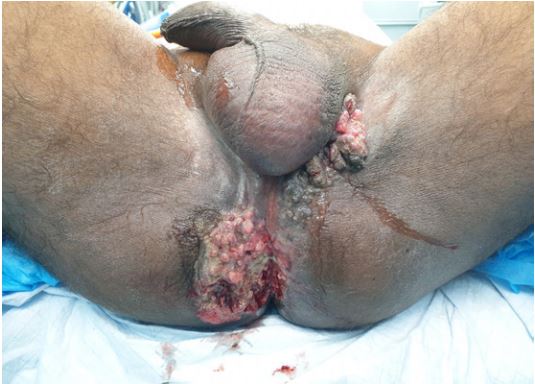
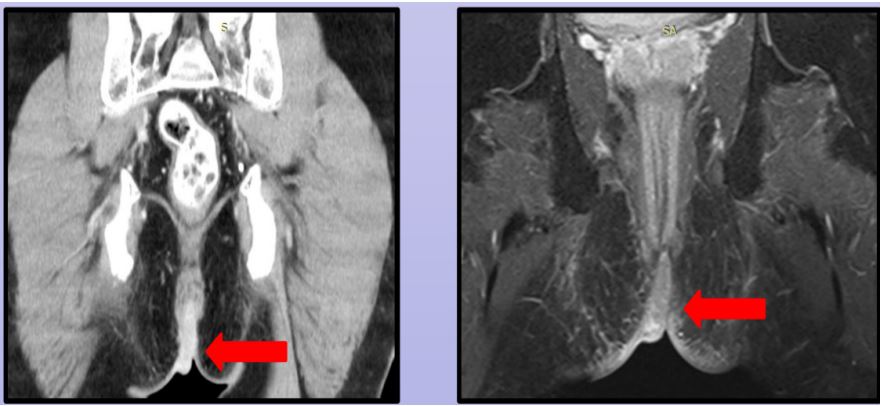
He presented 3 years later with recurrence of swelling over his perianal region. This time around the tumour was infiltrating into his anal canal, perineum and left hemi-scrotum (Figure 1). He was complaining of incontinence and severe pain over his perianal area. A repeat biopsy confirmed recurrence of squamous cell carcinoma. Clinically he also had palpable left inguinal lymph nodes. Staging Pelvic Magnetic Resonance Imaging (MRI) with staging CECT showed patient had enlarged left inguinal lymph nodes with no distant metastasis. Tumour was seen infiltrating into the anal canal (Figure 2); marked with arrow). Case was discussed in Multi-Disciplinary Oncology team and decision made for wide excision of the lesion. Pre operative planning to combine his surgery with the Plastic and Reconstructive team. We had a discussion with the infective diseases team to enquire on patient’s retroviral viral load and his fitness for surgery.His pre operative CD 4 count was 400 cells/mm3.
Patient underwent Laparoscopic assisted abdomino perineal resection, wide excision of peri anal tumour and V-Y plasty to achieve closure of wound (Figure 3, Figure 4, and Figure 5). Patient discharged home well on day 5 post operatively. We reviewed him 2 weeks following surgery, his wound healed well and patient was very happy. He has returned back to his work.He is on surveillance now with 3 monthly clinical assessment and annual CECT.
Figure 1
Figure 2: Routine pathological observation of tumor a. Tumor cells are arranged in bundles. b. Tumor cells are spindle-shaped or oval, with more lymphocytes and plasma cells infiltrated between cells. c. Vascular wall fibrin-like changes.
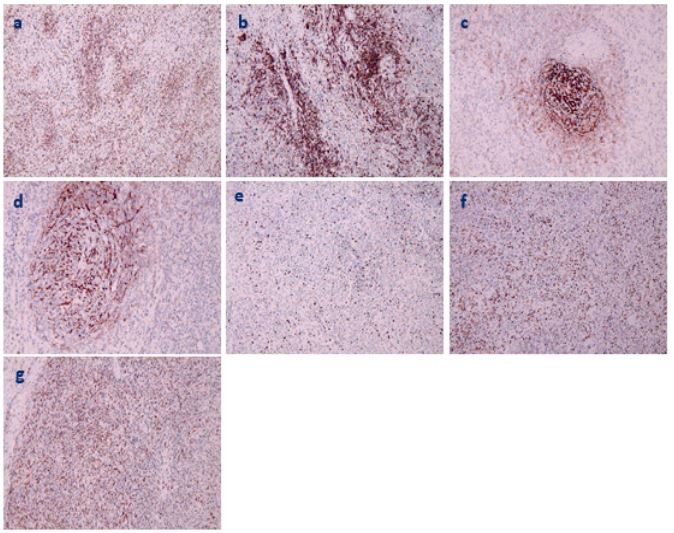
Figure 3: Immunohistochemical observation of tumor (magnification 100×, brown positive cells). a. CD3+. b. CD10+. c CD21+. d. CD35+. e. Ki-67 about 30%+. f. Mum-1+ .g. EBER+.
Discussion
The clinical manifestations are that the pathogenic mechanism of significant platelet reduction is very complex. Pathological changes in the spleen will lead to excessive platelet retention in the spleen, resulting in peripheral blood thrombocytopenia.
IPT-like FDCS is an extremely rare low-grade malignant tumor. Only 48 cases have been reported so far, with a male-to-female ratio of 1:1.5. Clinical manifestations include abdominal pain, bloating, abdominal mass, weight loss, fever, fatigue, anorexia, etc., but most cases have no obvious symptoms [7]. This case was reported for the first time with thrombocytopenia as the first symptom. The patient had no other symptoms except for bleeding gums and epistaxis.
The pathogenesis of IPT-like FDCS is related to EBV infection. It has also been documented that cases of IPT are associated with surgical or traumatic inflammation, autoimmune diseases, and infections such as Castleman disease and pallidococcus [8-11], although other pathogenic mechanisms remain unclear. This case was positive for EBV, accompanied by significant thrombocytopenia, suggesting that its pathogenesis was related to EBV infection and pathological changes in the spleen. Splenic tumor infiltration leads to splenic PLT retention, resulting in marked peripheral thrombocytopenia.
IPT-like FDCS consisted of neoplastic proliferative spindle cells with a large infiltrate of lymphoplasmacytic cells. The borders between cells are ill-defined and contain oval or elongated nuclei, vesicular or granular fine chromatin, and prominent nucleoli. Vascular fibrinoid changes are seen in this case, and EBV-induced cytokines and monokines are known to contribute to vascular proliferation, inflammation, and vascular injury [10], which may explain the vascular changes seen in our case.
Immunohistochemistry is crucial for the diagnosis of IPT-like FDCS. CD21 and CD35 are the preferred markers, positive support for the diagnosis of IPT-like FDCS. In this case, there are many plasma cells in the interstitium, CD138++, κ and λ light chains are all positive, CD43++, IgD-, and B-lineage gene rearrangement IGH and IGK monoclonal bands are all negative, so B-lineage lymphoma is excluded. About 30% of Ki-67 are positive, indicating that the tumor is low-grade malignant, and regular review and close attention are required.
In terms of treatment, surgical resection is usually the treatment of choice for patients with locally diseased tumors. The biological behavior of IPT-like FDCS is indolent, and patients can still survive for a long time after surgery. Survival for a long time even after relapse [12]. After splenectomy in this routine, platelet-raising therapy was given. After 17 months of follow-up, no tumor recurrence or metastasis was found, and the platelets returned to normal.
Conclusion
IPT-like FDCS is a clinically rare tumor. In this report, the cases of marked thrombocytopenia caused by the pathological changes of the spleen caused by the tumor are even rarer. The clinical and imaging manifestations of this tumor are non-specific, and the definitive diagnosis depends on histopathology and immunohistochemical staining. The biological behavior is indolent, and the prognosis is good after surgery and symptomatic treatment.
References
- Facchetti F, Simbeni M, Lorenzi L. Follicular dendritic cell sarcoma. Pathologica. 2021; 113: 316-329.
- Cheuk W, Chan JK, Shek TW, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol 2001; 25: 721-31.
- Morales-Vargas B, Deeb K, Peker D. Clinicopathologic and Molecular Analysis of Inflammatory Pseudotumor-Like Follicular/Fibroblastic Dendritic Cell Sarcoma: A Case Report and Review of Literature. Turk Patoloji Derg. 2021; 37: 266-272.
- Lifang Wang, Hui Deng, Mei Mao. Paraneoplastic pemphigus and myasthenia gravis, associated with inflammatory pseudotumor-like follicular dendritic cell sarcoma: response to rituximab. Clinical Case Reports 2016; 4(8): 797–799.
- Jia-Yi Zhuang, Fang-Fei Zhang, Qing-Wen Li, et al. Intra-abdominal inflammatory pseudotumor-like follicular dendritic cell sarcoma associated with paraneoplastic pemphigus: A case report and review of the literature. World J Clin Cases 2020 July 26; 8(14): 3097-3107.
- WANG Xiyan, LI Huiyuan, YANG Renchi. Immune Imbalance in Immune Thrombocytopenia. Chinese Journal of Cell Biology 2022; 44(1): 78-86.
- Bi-Xi Zhang, Zhi-Hong Chen, Yu Liu, et al. Inflammatory pseudotumor-like follicular dendritic cell sarcoma: A brief report of two cases. World J Gastrointest Oncol 2019 December 15; 11(12): 1231-1239.
- F Facchetti, P Incardona, S Lonardi, et al., Nodal inflammatory pseudotumor caused by leutic infection. American Journal of Surgical Pathology, 2009; 33( 3): 447–453.
- AC Tregnago, DL Morbeck, FD Costa, AH Campos, FA Soares and J Vassallo. Inflammatory pseudotumor-like follicular dendritic cell tumor: an underdiagnosed neoplasia. Applied Cancer Research, 2017; 37( 1): 45.
- Teruya-Feldstein J, Jaffe ES, Burd PR, et al. The role of Mig, the monokine induced by interferon-gamma, and IP-10, the interferongamma-inducible protein-10, in tissue necrosis and vascular damage associated with Epstein-Barr virus-positive lymphoproliferative disease. Blood 1997; 90: 4099-4105.
- Shuangshuang Deng, Jinli Gao. Inflammatory pseudotumor-like follicular dendritic sarcoma: a rare presentation of a hepatic mass. Int J Clin Exp Pathol 2019; 12(8): 3149-3155.
- Ge R, Liu C, Yin X, et al. Clinicopathologic characteristics of inflammatory pseudotumor-like follicular dendritic cell sarcoma. Int J Clin Exp Pathol 2014; 7: 2421-2429.