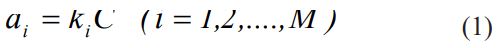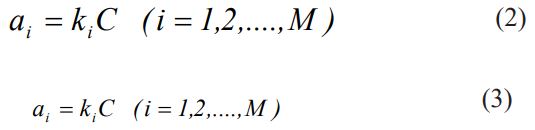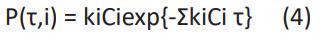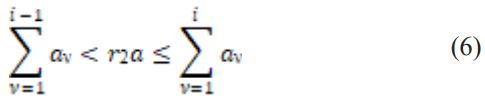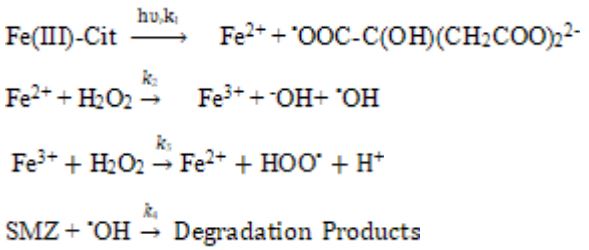Case Report - Volume 2 - Issue 5
Simulation removal of sulfamethazine, ciprofloxacin, sulfathiazole and amoxicillin via photo-Fenton process in by Monte Carlo modeling
Hamid Dezhampanah1,2*; Hamed Moradmand Jalali2
1Department of Chemistry, Faculty of Science, University of Guilan P.O. Box 1914, Rasht 0098, Iran.
2Department of Chemistry, University Campus 2, University of Guilan, Rasht, Iran.
Received Date : July 21, 2022
Accepted Date : Aug 25, 2022
Published Date: Sep 08, 2022
Copyright:© Hamid Dezhampanah 2022
*Corresponding Author : Hamid Dezhampanah, Department of Chemistry, Faculty of Science, University of Guilan, P. O. Box 41335-1914, Rasht 0098, Iran.Tel: + 98-13-33343631-5, Fax: + 98-131-3336762.
Email:h.dpanah@guilan.ac.ir
DOI: Doi.org/10.55920/2771-019X/1237
Abstract
Kinetic Monte Carlo modeling was employed as a powerful tool to kinetically investigate of antibiotics removal by photo-Fenton process (iron(III) citrate/ hydrogen peroxide in the presence of UV irradiation). Sulfamethazine, ciprofloxacin, sulfathiazole and amoxicillin are the antibiotics which were studied in this investigation. The kinetic mechanism of the photo-Fenton degradation was found by Monte Carlo simulation for several studied antibiotics including sulfamethazine, ciprofloxacin, sulfathiazole and amoxicillin. Also the rate constants values of each step in the suggested mechanism were acquired for these antibiotics through the simulation. Optimized values of Fe(III) citrate and hydrogen peroxide were attained through obtaining the effect of their initial amounts on the rate of antibiotics elimination by utilizing kinetic Monte Carlo simulation. The perfect agreement is observed between the simulation results and the experimental photo-Fenton data for the systems above.
Keywords: Kinetic; Monte Carlo; Antibiotic; Photo-Fenton; Simulation; Degradation.
Introduction
Antibiotics are principally prescription and consumed for treatment of bacterial infections in human and animals and furthermore used in livestock animal production as growth promoters [1]. Many antibiotics are negligibly metabolized in body [2,3], being excreted with their main component and transferred to wastewater. Existence of antibiotics in aquatic systems has become a main concern for scientists to discover efficient ways to eliminate these pollutants from water.
The elimination of antibiotics has been investigated in various platforms of water treatment. Recently advanced oxidation processes (AOPs) which are based on the hydroxyl radicals production, have been widely developed as the suitable methods for removing of non-biodegradable organic pollutants from water [4,5]. To the decomposition of antibiotics most studies have been done according to AOPs including photocatalytic oxidation [6,7], ozonation [8], Fenton and photo-Fenton [9-11]. In the Fenton process hydroxyl radicals is created from a solution of hydrogen peroxide and Fe (II) ions in an acidic media [12]. This method can be improved by the assistant of UV–vis irradiation (photo-Fenton process) that the reduction of Fe(III) to Fe(II) ions is hastened [13]. For example, degradation of sulfamethazine (SMZ), ciprofloxacin (CIP), sulfathiazole (STZ) and amoxicillin (AMX) in aqueous systems has been performed by photo-Fenton process (iron (III) citrate (FeCit)/H2O2/ UV irradiation) [14].
Monte Carlo (MC) modeling has been broadly used in different science such as engineering, physics, materials science and chemistry [15]. The MC method was used to study of structural properties in condensed phases comprising magnetic reactivity, thermophysical and mechanical properties [16–25]. Kinetic Monte Carlo (kMC) simulation has been widely employed as an excellent method to study of kinetic parameters in plentiful chemical process [26-33]. Kinetic Monte Carlo method has been magnificently developed to overcome some drawbacks of conventional deterministic modeling which can clearly contain atomic surface structure and reaction conditions as a function of time [34-37]. When a more accuracy is required in modeling, kMC simulation with outstanding efficiency is excellent for quick scans in diverse situations. In the kMC method, different mechanisms can be switched on and off via varying the diverse parameters and the influences of various factors on the output results can be easily investigated. By analysis different mechanistic states in this method and comparing the kMC consequences with studied experimental results, perception of the mechanisms is afforded. The kMC method has numerous superiorities to traditional method in solving numerically differential equations, which are more rapidly calculations for each step, facility of data handling, modeling of long time scales and temperature programing [38].
The main purpose of ongoing research is study kinetic parameters and mechanism of photo-Fenton process (FeCit/H2O2/UV) for removing antibiotics including SMZ, CIP, STZ and AMX from wastewater. The simulated concentration curves versus time were obtained for systems above of antibiotics and also the effects of various factors comprising such as initial concentrations of iron (III) citrate and H2O2 on the rate of antibiotics degradation were evaluated by kMC simulation.
Kinetic Monte Carlo modeling
The photo-Fenton process in removal of antibiotics including sulfathiazole, amoxicillin, sulfamethazine and ciprofloxacin [13] was kinetically simulated by the stochastic algorithm of the Monte Carlo technique [39]. Chemical Kinetic Simulator (CKS) software [40] was applied for the kinetic Monte Carlo (kMC) modeling.
In the algorithm of simulation, the reaction mechanism consists of several reactions, including:
The input information for kMC simulation is the steps of the supposed mechanism, the rate constants of each step (ki ), and the initial number of molecules in the reaction (Ci). Thus we have [39]
The reaction probability density function, P(τ,i), plays an important role in the algorithm of kMC modeling. P(τ,i) is calculated by Master equation [39]:
A random amount of τ (time of each step in mechanism) is achieved by drawing a random value (r1) from the constant distribution in the unit distance:
Moreover, a random value i can be caused by drawing a random amount (r2) from the uniform distribution in the unit interval by captivating i to be that value for which,
In this technique, r1 and r2 are created to compute τ and i through equations (5) and (6) [39].
The Monte Carlo simulation was extended via repeatedly random selecting among the probability-weighted steps in the suitable mechanism and updating the populations of reactants, and products according to the stoichiometric ratios for each step, state variables and reaction rates. Final resultants of the kMC modeling are curves of concentrations versus time. This stochastic numerical approach has been utilized to study various chemical systems [25-32]. In the ongoing research, kMC method was employed to kinetically simulation of STZ, AMX, CIP and SMZ degradation by photo-Fenton process.
Results and discussion
Perini and his colleagues were researched degradation of STZ, AMX, CIP and SMZ using photo-Fenton system containing iron (III) citrate/H2O2 at present UV irradiation and the concentration curves of each mentioned antibiotics versus time were attained [13]. By means of these experimental curves, a kinetically simulation of STZ, AMX, CIP and SMZ degradation by FeCit/H2O2/UV were performed. To perform kMC simulation, experimental information such as initial concentrations of antibiotics, FeCit and H2O2 and also temperature should be placed into CKS software as input data. The resultants of CKS simulation are concentration curves of antibiotics as a function of time should be in well agreement with existing experimental data.
In order to find the appropriate kinetic mechanism for antibiotics elimination by photo-Fenton system, at first the kMC modeling of experimental data were performed for SMZ degradation by FeCit/H2O2/UV. The input data for the simulation are steps of recommended mechanism, rate coefficients of each step and experimental reaction conditions (temperature= 298.15 K, initial concentration of SMZ=7.19×10-1 µM, initial concentration of FeCit =10 µM and initial concentration of H2O2=500 µM). Various mechanisms which have been proposed for antibiotic degradation by photo-Fenton system were simulated by kMC method. Most adjustable mechanism with existing experimental results were presented in this research. In the mechanism which has a perfect fitting with the experimental kinetic data, Iron (III) citrate is broken by UV irradiation and Fe2+ is created. Then oxidation of Fe2+ by H2O2 produces hydroxyl radicals and Fe3+. Produced Fe3+ reacts with hydrogen peroxide and Fe2+ is again generated that Fe2+/Fe3+ cycle is established. SMZ antibiotic degrades by created ●OH. These reactions are defined as below:
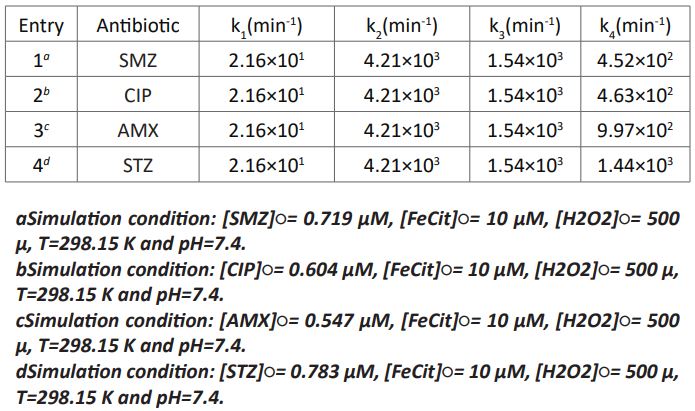
The accurate rate constants were determined by varying rate-determining step. Also the values of the rate constants of the aforementioned mechanism were changed up to a reasonable fitting between the simulated and experimental results [13] was found. The rate constants k1-k4 of the reactions 7-10 were achieved as variable parameters by Monte Carlo simulation as inserted in Table 1 (Entry 1). By using the offered mechanism and kMC simulation, the kinetic parameters for degradation of STZ, AMX and CIP by photo-Fenton process were also obtained. The adjustable rate coefficients of four steps for photo-Fenton decomposition of STZ, AMX and CIP antibiotics were recorded in entries 2, 3 of Table 1. As observed in this table, the step 1 with the rate constant k1 (reaction 7) is the rate-determining step in the destruction of all studied drugs by FeCit/H2O2/UV. Consequently, k1 is more significant than k3-k4 in the rate of the photo-Fenton process. As anticipated, the amounts of the rate constants k1-k3 are equal for the removal of SMZ, STZ, AMX and CIP but k4 is different in diverse antibiotics.
Table 1: Rate constants of simulated photo-Fenton mechanism for the removal of antibiotics.
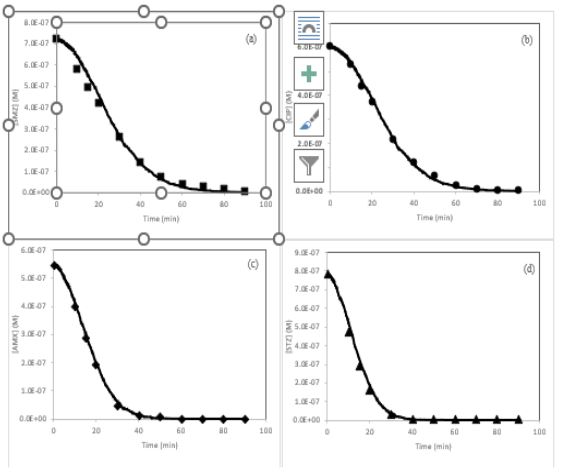
Figure 1: Kinetic data for removal of (a) SMZ, (b) CIP, (c) AMX and (d) STZ by photo-Fenton process. Experimental (Filled markers) and kMC simulation (solid line) results. Simulation condition: [SMZ]○= 0.719 µM, [CIP]○= 0.604 µM, [AMX]○= 0.547 µM, [STZ]○= 0.783 µM, [FeCit]○= 10 µM, [H2O2]○= 500 µ, T=298.15 K and pH=7.4.
Concentrations of STZ, AMX, SMZ and CIP versus time curves were shown as output results of CKS software for the simulated photo-Fenton (FeCit/H2O2/UV) process (Figure 1). As a result of this figure, simulated data shown as solid lines are in correct conformity with the experimental results [13] which were displayed as filled markers for all studied drugs. This Consistency proves that the advised mechanism can be proper for kinetically study of antibiotics degradation by photo-Fenton systems.
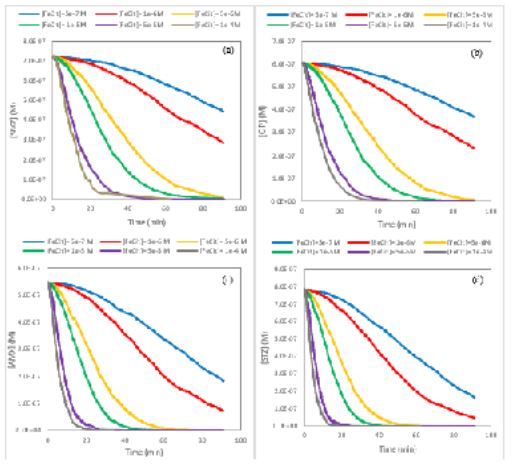
Effects of different conditions including initial amounts of FeCit and H2O2 on the removal rate of STZ, AMX, SMZ and CIP by the photo-Fenton process were investigated using the obtained kinetic parameters via kinetic Monte Carlo simulation. With the aim of the study, the effect of initial FeCit concentration on the rate of this process, different inlet iron (III) citrate concentrations (0.5, 1, 5, 10, 50 and 100 µM) were designated for simulations and inserted in CKS software as input data. Other inserted data for these simulations are temperature (298.15 K), initial concentration of antibiotics (200 µgL-1), initial amount of H2O2 (500 µM) [13], the gained steps of mechanism (reactions 10- 13) and the rate constants of steps (rate constants k1-k4, Table 1). (Figure 2) represents the kMC simulation curves of concentration versus times for the photo-Fenton degradation of STZ, AMX, SMZ and CIP by diverse initial FeCit concentrations. The rate of antibiotics degradation enhances by enhancement of initial FeCit amount as revealed in these curves. It can be said that optimized value of FeCit for the photo-Fenton removal of STZ, AMX, SMZ and CIP is 50 µM. In the presence of FeCit values greater than 50 µM, no significant increasing on the rate of photo-Fenton process was observed.
Figure 2: The removal rate of (a) SMZ, (b) CIP, (c) AMX and (d) STZ by various inlet concentration of FeCit. Simulation condition: [SMZ]○= 0.719 µM, [CIP]○= 0.604 µM, [AMX]○= 0.547 µM, [STZ]○= 0.783 µM, [H2O2]○= 500 µ, T=298.15 K and pH=7.4.
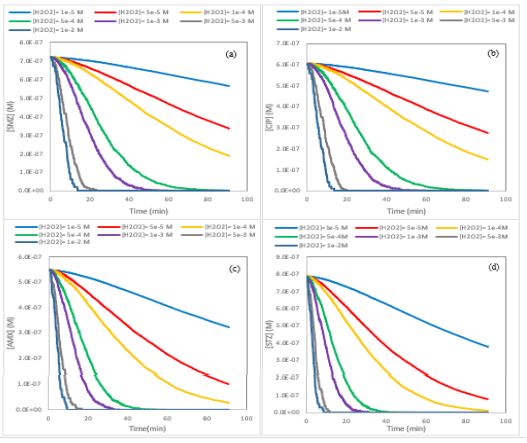
Also the influence of initial H2O2 value on the rate of STZ, AMX, SMZ and CIP degradation was investigated by obtained kinetic mechanism and parameters using kMC modeling. The initial amounts of hydrogen peroxide which were selected for this purpose are 1×10-5, 5×10-5, 1×10-4, 5×10-4, 1×10-3, 5×10-3 and 1×10-2 mol.L-1. For these simulations, the input information in CKS software are temperature (T=298.15 K), the reactions of obtained mechanism (reaction 7-10), the rate constants k1-k4 in (Table 1), initial concentration of antibiotics (200 µgL-1), initial concentration of FeCit (10 µM) and initial H2O2 amount and output results are antibiotics concentration as a function of times which depicted in (Figure 3). Clearly the rate of photo-Fenton process increases by increment of H2O2 amount.
Figure 3: KMC simulation data for photo-Fenton degradation of (a) SMZ, (b) CIP, (c) AMX and (d) STZ by different initial concentration of H2O2. Simulation condition: [SMZ]○= 0.719 µM, [CIP]○= 0.604 µM, [AMX]○= 0.547 µM, [STZ]○= 0.783 µM, [FeCit]○= 10 µM, T=298.15 K and pH=7.4.
Conclusion
To investigate the kinetics of photo-Fenton destruction was studied for antibiotics containing sulfamethazine, ciprofloxacin, sulfathiazole and amoxicillin by means of kMC simulation. The kinetic factors such as photo-Fenton mechanism and constant rate value of each step were determined using the kMC simulation. The effects of various parameters including initial concentrations of iron (III) citrate and H2O2 on the rate of above antibiotics degradation were investigated by the simulation. One of the advantages of this kMC study is obtaining optimized condition for photo-Fenton decay of antibiotics via a low-cost technique. The simulated outcomes show perfect fitting with the experimental photo-Fenton data for all studied antibiotics. Thus the offered mechanism is applicable for the kinetically research of photo-Fenton systems in pollutant removal from wastewater.
Acknowledgments
We gratefully acknowledge the graduate office of University of Guilan for supporting of this work.
Declarations
Funding: The authors declare no funding about this research.
Conflicts of interest/Competing interests: The authors declare that they have no conflict of interest.
Availability of data and material: The authors declare that there are data transparency.
Code availability: The authors declare that they have no software application or custom code.
Authors' contributions: All the authors have read and approved the final version (include appropriate statements).
References
- S Kim, D Aga. Potential ecological and human health impacts of antibiotics and antibiotic-resistant bacteria from wastewater treatment plants, J. Toxicol. Environ. Heal. Part B, 2007; 10: 559-573.
- K Kümmerer. Antibiotics in the aquatic environment −A review −Part I, Chemosphere, 2009; 75: 417–434.
- A Göbel, CS Mcardell, MJF Suter, W Giger. Trace determination of macrolide and sulfonamide antimicrobials, a human sulfonamide metabolite, and trimethoprim in wastewater using liquid chromatography coupled to electrospray tandem mass spectrometry, Anal. Chem. 2004; 76: 4756–4764.
- C Baeza, DRU Knappe. Transformation kinetics of biochemically active compounds in low-pressure UV Photolysis and UV/H2O2 advanced oxidation processes, Water Res. 2011; 45: 4531–4543.
- M Huber, S Canonica, YG Park, UV Gunten. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes, Environ. Sci. Technol. 2003; 3 (7): 1016–1024.
- Z Zhou, Z Shen, Z Cheng, G Zhang, M Li, Y Li, S Zhan, JC Crittenden. Mechanistic insights for efficient inactivation of antibiotic resistance genes: a synergistic interfacial adsorption and photocatalytic-oxidation process Sci. Bull. 2020; 65(24): 2107-2119.
- G Chen, Y Yu, L Liang, X Duan, R Li, X Lu, B Yan, N Li, S Wang. Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review, J. Hazard. Mater. 2021; 408: 124461.
- JM Sousa, G Macedo, M Pedrosa, C Becerra-Castro, S Castro-Silva, M Fernando, et al. Ozonation and UV254nm radiation for the removal of microorganisms and antibiotic resistance genes from urban wastewater, J. Hazard. Mater. 2017; 323: 434-441.
- MJL Claudia, GS Adrian, MTS Jose, CBL Madalena, MD Joaquim, L Faria. Homogeneous and heterogeneous photo-Fenton degradation of antibiotics using an innovative static mixer photoreactor, Chem. Eng. J. 2017; 310: 342-351.
- F Sopaj, N Oturan, J Pinson, FI Podvorica, MA Oturan. Effect of cathode material on electro-Fenton process efficiency for electrocatalytic mineralization of the antibiotic sulfamethazine, Chem. Eng. J. 2020; 384: 123249.
- S Sun, H Yao, W Fu, S Xue, W Zhang. Enhanced degradation of antibiotics by photo-fenton reactive membrane filtration, J. Hazard. Mater. 2020; 386: 121955.
- J Bandara, C Pulgarin, P Peringer, J Kiwi. Chemical (photo-activated) coupled biological homogeneous degradation of p-nitro-o-toluene-sulfonic acid in a flow reactor, J. Photochem. Photobiol. A Chem. 1997; 111: 253–263.
- J Pignatello, Dark and photoassisted iron(3+)-catalyzed degradation of chlorophenoxy herbicides by hydrogen peroxide, Environ. Sci. Technol. 1992; 26: 944–951.
- JA Lima Perini, AL Tonettib, C Vidal, CC Montagner, RFP Nogueira. Simultaneous degradation of ciprofloxacin, amoxicillin, sulfathiazole and sulfamethazine, and disinfection of hospital effluent after biological treatment via photo-Fenton process under ultraviolet germicidal irradiation, Appl. Catal. B: Environ. 2018; 224: 761–771.
- Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E. Equation of state calculations by fast computing machines. J. Chem. Phys. 1953; 21: 1087–1092.
- MP Allen, DJ Tildesley. Computer Simulation of Liquids. Oxford Science Publications, Oxford, 1989.
- D Frenkel, B Smit. Understanding Molecular Simulation: From Algorithms to Applications. Academic Press, New York, 1996.
- SM Auerbach. Theory and simulation of jump dynamics, diffusion and phase equilibrium in nanopores. Int. Rev. Phys. Chem. 2000; 19: 155-198.
- K Binder, Monte Carlo Methods in Statistical Physics, vol. 7. Springer, Berlin Heidelberg New York, 1986.
- K Binder. Atomistic modeling of materials properties byMonte-Carlo simulation, Adv. Mater. 1992; 4: 540-547.
- DP Landau, K Binder. A Guide toMonte Carlo Simulations in Statistical Physics. Cambridge University Press, Cambridge, 2020.
- G Ciccotti, D Frenkel, IR McDonald. Simulation of Liquids and Solids. Molecular Dynamics and Monte Carlo Methods in Statistical Mechanics. North-Holland, Amsterdam, 1987.
- DJ Dooling, LJ Broadbelt. Generic Monte Carlo tool for kinetic modeling. Ind. Eng. Chem. Res. 2001; 40: 522-529.
- GH Gilmer, HC Huang, TD Rubia, J Dalla Torre, F Baumann. Lattice Monte Carlo models of thin film deposition. Thin Solid Films, 2000; 365: 189-200.
- R Nieminen, A Jansen. Monte Carlo simulations of surface reactions, Appl. Catal. A: Gen. 1997; 160: 99–123.
- DR Alfonso, DN Tafen. Simulation of Atomic Diffusion in the Fcc NiAl System: A Kinetic Monte Carlo Study, J. Phys. Chem. C, 2015; 119: 11809-11817.
- L Liau, CY Lin. Vacancy defect distribution of colloidal particle deposition in a sedimentation process investigated using Kinetic Monte Carlo simulation. Colloids and Surfaces A: Physicochem. Eng. Aspects, 2011; 388: 70-76.
- H Moradmand-Jalali, Simulation of degradation of the organic contaminants ethylene glycol and phenol by iron nanoparticles using the kinetic Monte Carlo method. RSC Adv. 2014; 4: 32928-32933.
- H Bashiri. A new solution of Langmuir kinetic model for dissociative adsorption on solid surfaces, Chem. Phys. Lett. 2013; 575: 101-106.
- H Bashiri, H Moradmand-Jalali, H Rasa. Determination of intracellular levels of reactive oxygen species using the 2,7-dichlorofluorescein diacetate assay by kinetic Monte Carlo simulation, Prog. React. Kinet. Mec. 2014; 39: 281-291.
- H Moradmand-Jalali. Kinetic Investigation of Photo-Catalytic Activity of TiO2/metal nanocomposite in phenol photodegradation using Monte Carlo simulation, RSC Adv. 2015; 5: 36108–36116.
- H Moradmand-Jalali, H Bashiri, H Rasa. Study of photo-oxidative reactivity of sunscreening agents based on photo-oxidation of uric acid by kinetic Monte Carlo simulation, Mat. Sci. Eng. C, 2015; 50: 59–63.
- H Moradmand-Jalali. Kinetic study of antibiotic ciprofloxacin ozonation by MWCNT/MnO2 using Monte Carlo simulation, Mat. Sci. Eng. C, 2016; 59: 924–929.
- EW Hansen, M Neurock. First-principles-based Monte Carlo simulation of ethylene hydrogenation kinetics on Pd, J. Catal. 2000; 196: 241–252.
- DH Mei, EW Hansen, M Neurock. Ethylene hydrogenation over bimetallic Pd/Au (111) surfaces: application of quantum chemical results and dynamic Monte Carlo simulation, J. Phys. Chem. B, 2003; 107: 798–810.
- M Neurock, DH Mei. Effects of Alloying Pd and Au on the Hydrogenation of Ethylene: An ab initio-Based Dynamic Monte Carlo Study, Top. Catal. 2002; 20: 5–23.
- D Mei, PA Sheth, M Neurock, CM Smith. First-principles-based kinetic Monte Carlo simulation of the selective hydrogenation of acetylene over Pd (111), J. Catal. 2006; 242: 1–15.
- Cuppen HM, Karssemeijer LJ, Lamberts T. The kinetic Monte Carlo method as a way to solve the master equation for interstellar grain chemistry. Chem Rev. 2013; 113(12): 8840–8871.
- DT Gillespie. A general method for numerically simulating the stochastictime evolution of coupled chemical reaction, J. Comp. Phys. 1976; 2: 403-434.
- IBM, CSK Chemical Kinetics Simulator 1.01, IBM Almaden Research Center; IBM Corporation 1995.