Case Report - Volume 2 - Issue 5
Iatrogenic Segmental Renal Artery Pseudoaneurysm: A Case Report
Lorenzo Capone1*; Giovanni Ciccarese2; Antonio Cisternino1
1Department of Urology, Fondazione IRCCS Casa Sollievo Della Sofferenza, San Giovanni Rotondo, Italy.
2Department of Interventional Radiology, Fondazione IRCCS Casa Sollievo Della Sofferenza, San Giovanni Rotondo, Italy
Received Date : Aug 22, 2022
Accepted Date : Sep 15, 2022
Published Date: Sep 26, 2022
Copyright:© Lorenzo Capone 2022
*Corresponding Author : Lorenzo Capone, Department of Urology, Fondazione IRCCS Casa Sollievo Della Sofferenza, San Giovanni Rotondo,Italy.
Email:lorenzocapone@msn.com
DOI: Doi.org/10.55920/2771-019X/1241
Introduction
Pseudoaneurysm is a false aneurysm that occur at the site ofarterial injury that involves one or more layers of the arterialwall but no all three layers of the wall [1]. The renal artery pseudoaneurysm is a rare clinical entity due to iatrogenic procedures like percutaneous procedures, renal biopsy, nephrectomy or to penetrating or blunt traumas [2,3]. The clinical can be different according to patient blood pressure, blood flow and also to the effectiveness of the hemostasis. This complication can lead to hematuria, blood loss, and even hemorrhagic shock secondary to rupture (rare event but with a mortality rate as high as 80%) [4]. Aneurysms larger than 2 cm in diameter are considered to have a high risk of rupture, although ruptures have also been reported in smaller aneurysm [5].
Case report
We present the case of a 56-year-old female patient admitted to the orthopedic department for scoliosis and undergoing L3 - L4 arthrodesis surgery. The operation was interrupted due to bleeding on the left flack side and the thoracic surgeon was urgently called to evaluate the presence of trauma to the diaphragm and mediastinum. Despite the thoracic evaluation, the patient continued to have hemoglobin loss and worsening of renal function indices (Hemoglobin 7 gr/dL from 10 gr/ dL; Creatinine 1.50 mg/dL from 0.80 mg/dL; Azotemia 55 mg/ dL from 31 mg/dL). For these reasons a CT scan of the abdomen and pelvis with contrast was performed. On baseline examination there was total edematous imbibition of the left peri-renal fat. After injection of iodinated contrast, a mesorenal fracture was found at the level of the upper pole. Finally, during late urographic scans at 5 and 15 minutes, the contrast was observed to spread from the upper renal calyces to the posterior renal space, forming a urinoma (Figure 1,2). The patient immediately underwent endoscopic surgery for placement of mono-j ureteral stent and bladder catheter with drainage of clots at bladder level. The next CT scan after six days documented reduced contrast leakage at the level of the left upper pole renal laceration, almost complete resorption of the urinoma, and the distal end of the ureteral stent correctly positioned near the upper calyces (Figure 3). Nevertheless, blood loss and gross hematuria persisted the next day, so it was decided to perform angiography.
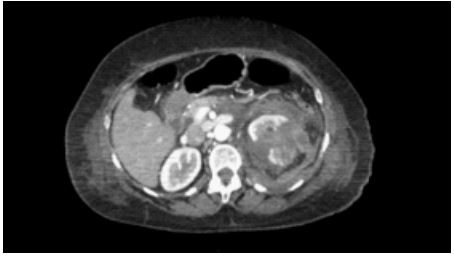
Figure 1: Tac showing meso-renal fracture at the level of the left upper pole.
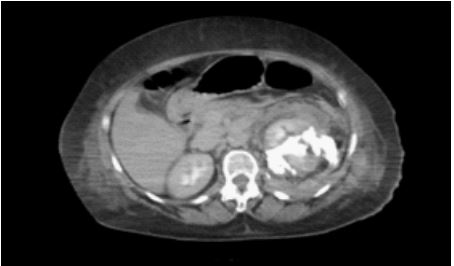
Figure 2: Tac showing a urinoma
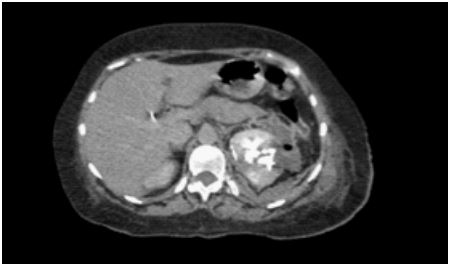
Figure 3: Tomography situation after 6 days with a double j uretheral stent
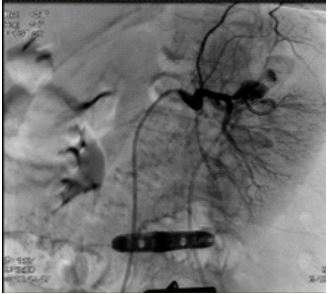
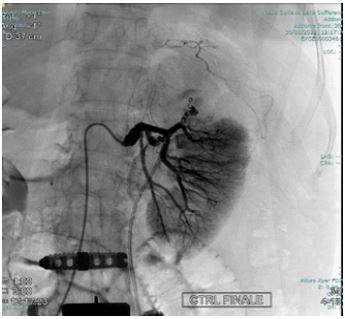
Figure 4: 2 Pseudoaneurysms in correspondence of the superior and mesorenal renal pole.
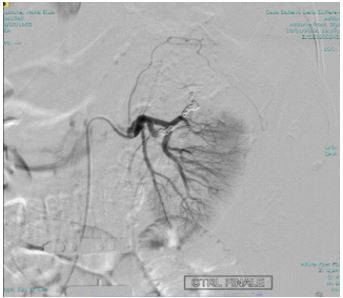
Angiography was performed using a right transfemoral arterial approach with study of the abdominal aorta, selective catheterization of the left renal artery, and super selective intraparenchymal branches of the middle and upper renal pole using a microcatheter under anesthesia care. The examination revealed the presence of two spreads of constraint of the renal parenchyma compatible with pseudoaneurysms in correspondence of the superior and mesorenal renal pole, both less than 2 centimeters (Figure 4). Finally, embolization of the arterial branches afferent to the lesions was performed by positioning metal coils with controlled release (3 at the upper pole: 2 x 70 mm and 1 x 70 mm; 2 at the mesorenal level of 3 x 70 mm) (Figure 5,6). In the days following the embolization there was a rise in hemoglobin, the complete disappearance of hematuria and a normalization of the indexes of phlogosis
Figure 5: Coil embolization.
Figure 6: Coil embolization.
Discussion
A true aneurysm is a circumscribed dilatation of an artery that is surrounded by the intima, media, and adventitia. A pseudoaneurysm, by contrast, forms as a result of an injury to one or more layers of the arterial wall [1,2,6]. These vascular lesions are generally related to renal biopsy, nephrectomy, renal transplantation, or percutaneous procedures. In addition, there is a re¬lationship with penetrating traumas and, more rarely, with blunt traumas (the mechanism of sudden decel¬eration in automobile accidents is the most probable cause) [2,3,7]. Renal artery pseudoaneurysm is a rare but serious condition because it involves an arterial perforation that is occluded only by hematoma and connective tissue with a high propensity for rupture [4,5]. A controlled bleed, in fact, can become a life-threatening hemorrhage if the balance between the tamponade effect of the surrounding hematoma and connective tissue, and the intraluminal hydrostatic pressure changes [8].When there is rupture, there are four spaces the blood can be redistributed: retroperitoneal, intraperitoneal, intrarenal and intrapelvic; fortunately most intraparenchymal renal artery pseudoaneurysm ruptures are self-contained. Symptoms may include abdominal tenderness, abdominal mass, hematuria, hypertension until shock. In renal artery pseudoaneurysm hematuria is the most common symptom because of the pseudoa¬neurysm erosion to the adjacent renal collection system. This situation can lead to worsening of kidney function, anuria and dialysis [6,7,9].
Diagnosis of renal pseudoaneurysm can be complicated by a lack of specific clinical signs and symptoms or by a failure to identify potential mechanisms of arterial injury. When the patient is hemodynamically stable, the non-invasive examinations of choice are doppler ultrasound, computer tomography, and magnetic resonance imaging. Early detection and treatment of renal pseudoaneurysm is important to avoid potential morbidity from this condition [6].
Treatment of renal pseudoaneurysm consists of nephrectomy, open vascular surgery, or angiographic embolization, depending on the patient’s clinical condition. Angioembolization is the currently accepted first line therapy for renal pseudoaneurysms because of its greater success rate of up to 80% and lower complication rate compared to those of surgical approaches [5,6]. If interventional radiology is not available or if substantial delay in getting a patient to angiography is expected, surgery is the alternative (evacuation and closure of the arterial defect, or evacuation and ligation of the offending artery) [10,11]. There are no established protocols for posttreatment follow-up of patients who have undergone selective angioembolization, but it is, however, generally accepted among experts that the patient should be closely monitored by both laboratory and first and second level diagnostic tests, 24 hours apart, one week and then one month after surgery [5,11].
References
- Sueyoshi E, Sakamoto i, Nakashima K, Minami K & Hayashi K. visceral and peripheral arterial pseudoaneurysms. AJR Am.J. Roentgenol. 2005; 185: 741–749.
- Franklin JA, Brigham D, Bogey wM & Powell CS. Treatment of iatrogenic false aneurysms. J. Am. Coll. Surg. 2003; 197:293–301.
- Tsutsui A, Hasegawa S, Andou S. Renal artery pseudoaneurysm after open partial nephrectomy: a case report. Hinyokika Kiyo. 2007 Feb;53(2):125-8. PMID: 17352164.
- Halachmi S, Chait P, Hodapp J, et al. Renal pseudoaneurysm after blunt renal trauma in a pediatric patient: Management by angiographic embolization. Urology. 2003; 61: 224.
- Mima A, Toma M, Matsubara T, Shiota F, Iehara N, Abe H, et al. Angio-embolization of renal artery pseudoaneurysm after renal biopsy: a case report. Ren Fail. 2009; 31(8): 753-5.[PMID: 19814646 DOI: 10.3109/08860220903125298].
- Ngo TC, Lee JJ, Gonzalgo ML. Renal pseudoaneurysm: an overview. Nat Rev Urol. 2010 Nov; 7(11): 619-25. [PMID: 20938436 DOI: 10.1038/nrurol.2010.163].
- Roman LI, Efel CF, França VT, Merten CM, Dummer CD. Renal artery pseudoaneurysm. J Bras Nefrol. 2017 Oct-Dec; 39(4): 458-461. English, Portuguese. [PMID: 29319774 DOI:10.5935/0101-2800.20170080].
- Negoro H, Kawakita M, Koda Y. Renal artery pseudoaneurysm after laparoscopic partial nephrectomy for renal cell carcinoma in a solitary kidney. Int J Urol. 2005 Jul; 12(7): 683-5. [PMID: 16045563 DOI: 10.1111/j.1442-2042.2005.01130.x].
- Zorn KC, Starks CL, Gofrit ON, Orvieto MA, Shalhav AL. Embolization of renal-artery pseudoaneurysm after laparoscopic partial nephrectomy for angiomyolipoma: case report and literature review. J Endourol. 2007 Jul; 21(7): 763-8. [PMID:17705767 DOI: 10.1089/end.2006.0332].
- Cohenpour M, Strauss S, Gottlieb P, Peer A, Rimon U, Stav K, Gayer G. Pseudoaneurysm of the renal artery following partial nephrectomy: imaging findings and coil embolization. Clin Radiol. 2007 Nov; 62(11): 1104-9. [PMID: 17920871 DOI:10.1016/j.crad.2007.06.004].
- Kisa E, Koc G, Yucel C, Keskin MZ, Capar AE, Kozacioglu Z. Renal artery pseudoaneurysm after open partial nephrectomy for renal cell carcinoma. Urologia. 2020 Feb; 87(1): 11-14. [PMID: 31280692 DOI: 10.1177/0391560319862250].