Case Report - Volume 2 - Issue 5
Ectopic-Adrenocorticotropic Hormone secretion in adolescents and young adult patients with neuroendocrine neoplasm: a challenge for clinical and social management
Alice Laffi1*; Andrea Gerardo Antonio Lania2,3; Elisabetta Lavezzi2; Marcello Rodari4; ittorio Pedicini5; Alessandro Zerbi6, 3; Silvia Carrara7; Paola Spaggiari8; Carlo Carnaghi9 nd Alexia Francesca Bertuzzi1
1Medical Oncology and Hematology Unit, IRCCS Humanitas Research Hospital, Via Manzoni 56, Rozzano, 20089 Milan, Italy.
2Endocrinology, Diabetology and Andrology Unit, IRCCS Humanitas Research Hospital, Rozzano, Italy.
3Department of Biomedical Sciences, Humanitas University, Rita Levi Montalcini 4, Pieve Emanuele, 20072 Milan, Italy.
4Unit of Nuclear Medicine, IRCCS Humanitas Research Hospital, Via Manzoni 56, Rozzano, 20089 Milan, Italy.
5Radiology Unit, IRCCS Humanitas Research Hospital, Via Manzoni 56, Rozzano, 20089 Milan, Italy.
6Pancreatic Surgery, IRCCS Humanitas Research Hospital, Via Manzoni 56, Rozzano, 20089 Milan, Italy.
7Endoscopy Unit, Department of Gastroenterology, IRCCS Humanitas Research Hospital, Via Manzoni 56, Rozzano, 20089 Milan,Italy.
8Department of Pathology, IRCCS Humanitas Research Hospital, Via Manzoni 56, Rozzano, 20089 Milan, Italy.
9Medical Oncology, Humanitas Istituto Clinico Catanese, Catania, Sicilia, Italy
Received Date : July 04, 2022
Accepted Date : Sep 01, 2022
Published Date: Sep 15, 2022
Copyright:© Alice Laf 2022
*Corresponding Author : Alice Laffi, IRCCS Humanitas Research Hospital,via Manzoni 56, 20089, Rozzano, Milan, Italy.Tel: 0282243162
Email:alice.laffi@humanitas.it
DOI: Doi.org/10.55920/2771-019X/1244
Introduction
Well and poorly differentiated neuroendocrine neoplasms (NENs) may be associated with an ectopic production of Adrenocorticotropic Hormone (ACTH) [1] that leads to a paraneoplastic Cushing Syndrome (CS). This heterogeneous clinical pattern is the result of a hypercortisolism [2] due to an uncontrolled and continuous hyperstimulation of the adrenal cortex, which escapes the negative feedback of the Hypothalamus-Hypophysis axis [3]. Among NENs, ectopic-ACTH secretion (EAS) has been historically associated with small-cell lung cancers (SCLCs), but the improvement of the knowledge and diagnostic techniques led to a broadening of the causes also to NENs with different morphologies and from other sites [4]. According to the previous published data, EAS by gastro-entero-pancreatic (GEP) NENs occurs in almost 13% of cases. Irrespective of the NENs site of origin and the biology (which means morphology and proliferation index), the CS may represent a life-threatening condition for the patients due the consequences of the mineralocorticoid effect of persistent high levels of cortisol. These patients may experience a plethora of different symptoms which includes diffuse oedema, overweight/obesity of the trunk and striae rubrae, but also severe hypokalemia and hypertension, that represent medical emergencies and tumor fatal consequences even without a progression disease [1]. EAS may initially occur with non-specific clinical manifestations, as psychiatric complaints, recurrent orthopedic issues, sexual and reproductive disfunctions [3] that are not easily related to a tumoral cause and lead the patient presents to many specialists before being referred to an endocrinology / oncology Center. For all these reasons, CS diagnosis is often delayed. In a very small pool of cases [5], EAS may occur in the peculiar category of the adolescents and young adults (AYA) patients, worldwide defined as the cancerpopulation between 15 and 39 years of age [6]. Generally, the cancer diagnosis in AYA population is delayed, on one hand due to the sense of invulnerability and the minimizing of the symptoms of AYA patients and, on the other, the trend of the providers to a low suspicion of cancer in AYA population. Therefore, the features of the AYA category together with the equivocal clinical patterns of CS may lead to a delay of the diagnosis and management of EAS by extra-pituitary NENs [3, 7]. Here we reported the case of a NEN AYA patient with CS by EAS and a review of the literature on this topic in this peculiar population.
Methods
A critical review of published data about the EAS topic related to NENs has been performed on the PubMed database. Eligibility criteria for the selection included case reports and case series of patients between 15-39 years of age with a proven EAS by a NEN; reviews, letters to the Editor and retrospective analyses were included too. The keywords were: adolescents AND AYA AND neuroendocrine tumor AND ectopic-ACTH. All studies on EAS in not-AYA patients or not related to NENs were excluded. Radiological, biochemical, and pathological data about the clinical case treated at our Institute were collected retrospectively according to the Helsinki Declaration.
Case report and Literature review
A 32-years old woman with a history of almost 1 year of periocular dermatitis, stomatitis, gastritis, and a suspicious nodular erythema, went to the Emergency Room (ER) with a sudden increase of the abdominal volume, oedema, and pain of the limbs. Blood examinations showed an increase of transaminases, amylase, and lipase, raising suspicion of acute pancreatitis. A CT scan revealed a solid mass of the pancreatic body-tail, suspected to be a primary tumor, associated with multiple abdominal lymphadenopathies, liver metastases and ascites (Figure 1). An esophago-gastro-duodenoscopy showed a diffuse gastropathy and duodenitis with multiple duodenal ulcers (Figure 2A). Endoscopic Ultrasound (EUS) confirmed the pancreatic mass surrounded by lymphadenopaties. An EUSguided Fine Needle Biopsy (FNB) was performed with acquisition of tissue cores from both the mass and lymph-nodes and led to the diagnosis of a well-differentiated NET with a Ki-67 of 13%, grade 2 (G2) according to the 2019 WHO classification (8) (Figure 2B and 2C). The immunohistochemistry showed a positivity in Synaptophysin, Chromogranin A, Gastrin expression (Figure 3). Blood exams revealed high levels of gastrin (625 pg/mL - normal range 25 - 111), leading to clinically classify as Gastrinoma the pancreatic NET. The staging was completed with 68Gallium-DOTATOC and a 18FDG-PET/CT scan (Figure 4). The dual-tracers imaging showed a great Somatostatin Receptors (SSRT) expression on the pancreatic lesion, on abdominal lymphadenopathies and on liver metastases; a mild FDG uptake on pancreatic lesion and on lymphadenopathies was detected while a high metabolism was seen on the S6 liver metastasis. An endocrinologic assessment revealed high levels of ACTH and Cortisol (respectively 185 pg/mL - normal range 5 - 50, and 23.1 ug/dL - normal range 6.7 - 22.6). In the suspicion of an ectopic ACTH syndrome, a High dose dexamethasone suppression test was performed thus confirming the diagnosis of CS by an ACTH-secreting pancreatic NET.
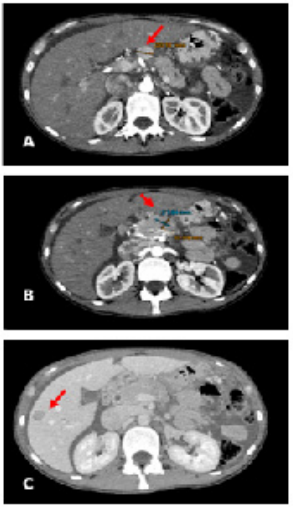
Figure 1: CT scan of the abdomen
A: CT scan of the abdomen. The red arrow showed lymphadenopathy.
B: CT scan of the abdomen. The red arrow showed the lesion of the pancreas
C: CT scan of the abdomen. The red arrow showed a liver metastasis.
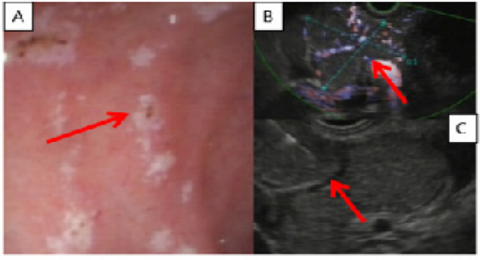
Figure 2: Esophaguo-gastro-duodenoscopy and Endoscopic Ultrasound (EUS)-guided Fine Needle Biopsy
A: The endoscopy shows the duodenal ulcers (red arrow)
B: The EUS detects the hypervascularized pancreatic mass (red arrow)
C: EUS-guided FNB of peripancreatic lymphadenopathy (red arrow)
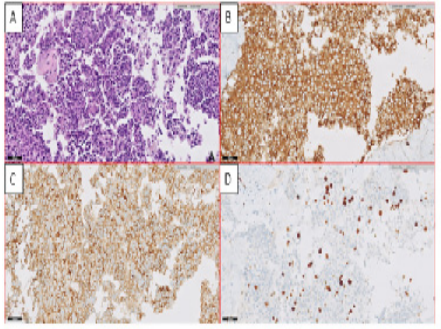
Figure 3: Pathologic features of the neuroendocrine tumor collected by biopsy
A: Well-differentiated morphology of the neuroendocrine tumor: hematoxylin and eosin
B: Synaptophysin expression
C: Gastrin expression
D: Immunostaining of Ki-67
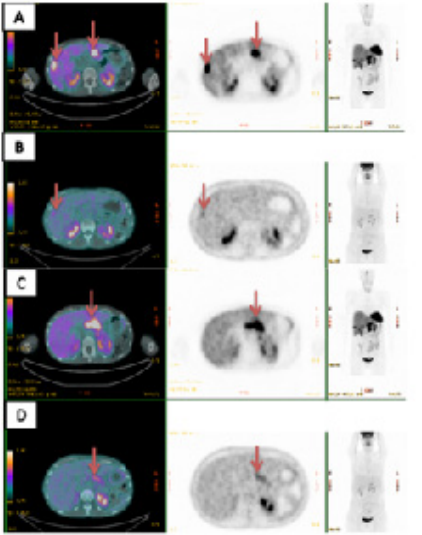
Figure 4: Functional imaging as staging of the pancreatic Neuroendocrine Tumor.
A: 68Gallium-DOTATOC PET/CT scan positivity on liver metastases (red arrows)
B: The mild 18FDG uptake on a single liver metastasis (red arrow)
C: 68Gallium-DOTATOC PET positivity on pancreatic lesion (red arrow)
D: The absence of 18FDG uptake in the FDG PET/CT scan on the lesion of the pancreas (red arrows)
According to the stage and the histology, a systemic treatment with long-lasting somatostatin analogue (SSA) was started in November 2020 with an initial ACTH level decrease and an improvement of the syndrome. After 2 months, the patient was admitted in the ER due to an ophthalmic infection by Herpes Zoster associated with headache, mental confusion, and disorientation. The Severe Acute Respiratory Syndrome (Sars)-Coronavirus (Cov) -2 test revealed a systemic COVID-19 infection, and the patient was admitted in the Department of Infectious Diseases. Since a brain Magnetic Resonance imaging (MRI) excluded distant metastases, a spinal tap was performed and confirmed a Varicella Zoster Virus (VZV) related Encephalitis. The patient was treated with Acyclovir with an improvement of the neurological symptoms. Due to a worsening of the CS, Metyrapone therapy (up to 1000 mg/day) was started with a quick improvement of biochemical levels of ACTH/cortisol and clinical signs and symptoms. The endocrinologic assessment of bone metabolisms revealed a severe osteoporosis with initial morphometric signs of vertebral fractures that prompted us to start an osteoactive treatment (i.e. Zolendodronic acid 5 mg ev). Moreover, multiple abscesses of the limbs were identified, and the patient underwent systemic antibiotics and surgical debridement.
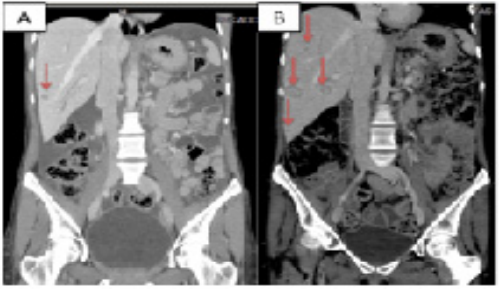
Figure 5: CT scan of the liver progression disease over Somatostatin analogue therapy (SSA).
A: CT scan performed at the SSA start (red arrow shows the liver lesion)
B: CT scan performed after 5 months SSA start shows the liver progression disease (red arrows).
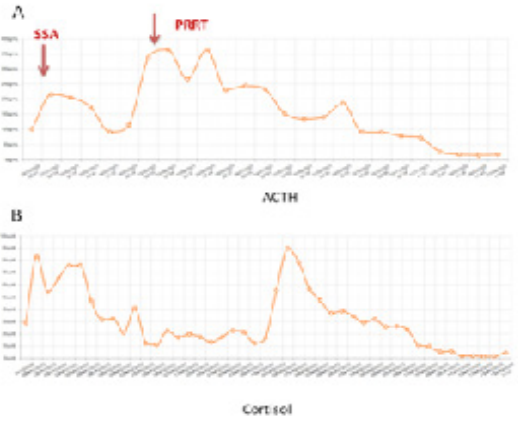
Figure 6: Trend of Adrenocorticotropic Hormon (A) and Cortisol (B) over the treatments
(SSA: Somatostatin analogue; PRRT: Peptide Radioligand Receptor Therapy)
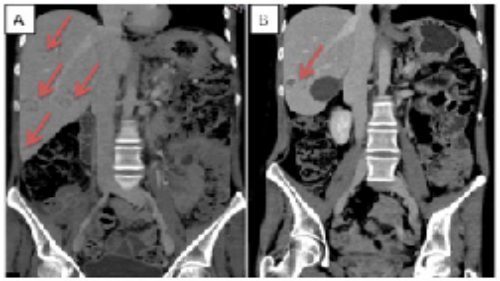
Figure 7:T Partial response after Peptide Radioligand Receptor Therapy (PRRT)
A: CT scan after Somatostatin analogue (SSA) progression disease and before the PRRT start (red arrows showed the liver metastases)
B: CT scan performed after PRRT confirms the partial response to the treatment (red arrow shows the liver metastasis)
After the discharge, in April 2021, a further CT scan showed a progressive disease of liver metastases (Figure 5). According to the positivity to 68Gallium-DOTATOC PET/CT scan, a Peptide Radionuclide Receptor Therapy (PRRT) was proposed. After 2 177Lu-DOTATATE administration (7400 MBq respectively), the treatment was stopped due to a new osteomyelitis and the patient underwent a further sub-astragalic arthrodesis and surgical debridement. In Summer 2021, ACTH and cortisol levels progressively increased despite the up titration of Metyrapone dosage thus prompting the Endocrinologists to switch to Osilodrostat 5 mg /day, another inhibitor of the cortisol synthesis through the interaction with 11β-hidroxilase (CYP11B1), that quickly normalized hormone secretion. Considering the clinical and biochemical effects of PRRT on CS and evaluating the case within the NET-dedicated multidisciplinary team (MDT), the patient completed the treatment with the last 2 administrations of 177Lu-DOTATE (Figure 6). After PRRT, the patients continued the regular administration of Lanreotide 120 mg / 4 weeks, while two further orthopedic surgeries were performed in November 2021 and January 2022. In April 2022, a restaging CT scan showed a partial morphologic response of distant lesions with a stability of the receptorial expression (Figure 7).
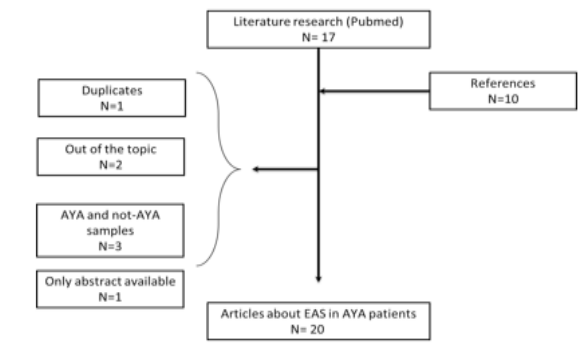
Figure 8: CONSORT flow chart of the literature research.
AYA: adolescents and young adults; EAS: ectopic-ACTH secretion
Discussion
The incidence of EAS in adult age accounts for almost 10-15% of CS, while the EAS related to extra-pituitary NENs is currently unknown [5]. The largest analyses reported a prevalence of 3.2% among thoracic and GEP NENs [9], while CS by paraganglioma/pheochromocytomas are mostly anecdotal [10]. Data on the incidence of the EAS by NENs in younger patients are lacking, with the exception for small and retrospective case series that cannot be considered representative of the whole AYA subgroup. Furthermore, it is likely to think that paraneoplastic CS misdiagnosis or diagnosis delay might be even longer in AYA than adult patients due to the their typical sense of invulnerability to diseases, that leads to underestimate the cancer-related symptoms, and the provider’s trend to not suspect oncologic diseases in the AYA population [6]. Actually, the diagnostic delay of the case we presented and those collected by the literature review seems to be not so different to the result of a recent meta-analysis by Rubistein et al on the time of CS by EAS diagnosis (a mean of 14 months) [11]. Unfortunately, all the 18 papers selected in meta-analysis carried out a comprehensive analysis, without performing a subgroup analysis between AYA, pediatric and adult patients. Thus, the diagnostic delay has yet to be demonstrated in AYA population.
Conclusion
EAS linked to extra-pituitary NENs in AYA patients represents a rare but challenging clinical and psychosocial issue that may engage many medical figures and not. Our case report and the literature review seem to suggest the importance of education and awareness programs targeted to practitioners, families and AYA peers to reduce the diagnostic/therapeutic delay of cancer and the impact of its consequences on AYA patients’ health.
Financial support: None.
Conflicting interests: The Authors declare that there is no conflict of interest.
Funding: This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent: Written informed consent was collected for retrospective analyses according to the Humanitas Research Hospital Recommendations. The study was conducted in accordance with Helsinki Declarations.
Ethical approval: This retrospective analysis of a case complies with the Declaration of Helsinki and, according to the ethical approval of the Humanitas Research Hospital, the Ethical Approval is not required. Contributor ship: A.L. and A.F.B and A.G.A.L.: researching and conceptualization of the analysis, writing and reviewing the results; A.L., A.F.B, A.G.A.L., E.L., M.R., V.P., A.Z., S.C., P.S., C.C.:
reviewing the analysis and the language.
References
- Young J, Haissaguerre M, Viera-Pinto O, Chabre O, Baudin E, Tabarin A. MANAGEMENT OF ENDOCRINE DISEASE: Cushing’s syndrome due to ectopic ACTH secretion: an expert operational opinion. Eur J Endocrinol. 2020; 182(4): R29-R58.
- Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014; 4(2): 739-69.
- Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing’s syndrome due to ectopic corticotropin secretion:twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005; 90(8): 4955-62.
- Liddle GW, Nicholson WE, Island DP, Orth DN, Abe K, Lowder SC. Clinical and laboratory studies of ectopic humoral syndromes. Recent Prog Horm Res. 1969; 25: 283-314.
- More J, Young J, Reznik Y, Raverot G, Borson-Chazot F, Rohmer V, et al. Ectopic ACTH syndrome in children and adolescents. J Clin Endocrinol Metab. 2011; 96(5): 1213-22.
- Alliance USDOHAHSNIoHNCILYA. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer Report of the Adolescent and Young Adult Oncology Progress Review Group. 2020.
- Trama A, Botta L, Foschi R, Ferrari A, Stiller C, Desandes E, et al. Survival of European adolescents and young adults diagnosed with cancer in 2000-07: population-based data from EUROCARE-5. Lancet Oncol. 2016; 17(7): 896-906.
- Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020; 76(2): 182-8.
- Kamp K, Alwani RA, Korpershoek E, Franssen GJ, de Herder WW, Feelders RA. Prevalence and clinical features of the ectopic ACTH syndrome in patients with gastroenteropancreatic and thoracic neuroendocrine tumors. Eur J Endocrinol. 2016; 174(3): 271-80.
- Daya R, Wingfield C, Sotshononda P, Seedat F, Bulbulia S, Simmons MD, et al. Ectopic Cushing’s Syndrome Secondary to Metastatic Paraganglioma. Case Rep Endocrinol. 2021; 2021: 5593920.
- Rubinstein G, Osswald A, Hoster E, Losa M, Elenkova A, Zacharieva S, et al. . Time to Diagnosis in Cushing’s Syndrome: A Meta-Analysis Based on 5367 Patients. J Clin Endocrinol Metab. 2020 Mar 1; 105(3)
- Alexandraki KI, Grossman AB. Therapeutic Strategies for the Treatment of Severe Cushing’s Syndrome. Drugs. 2016;76(4): 447-58.
- Mancini T, Kola B, Mantero F, Boscaro M, Arnaldi G. High cardiovascular risk in patients with Cushing’s syndrome according to 1999 WHO/ISH guidelines. Clin Endocrinol (Oxf). 2004; 61(6): 768-77.
- Hasenmajer V, Sbardella E, Sciarra F, Minnetti M, Isidori AM, Venneri MA. The Immune System in Cushing’s Syndrome. Trends Endocrinol Metab. 2020; 31(9): 655-69.
- Sarlis NJ, Chanock SJ, Nieman LK. Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropin. J Clin Endocrinol Metab. 2000; 85(1): 42-7.
- Bakker RC, Gallas PR, Romijn JA, Wiersinga WM. Cushing’s syndrome complicated by multiple opportunistic infections. J Endocrinol Invest. 1998; 21(5): 329-33.
- Feldman BJ. Glucocorticoids influence on mesenchymal stem cells and implications for metabolic disease. Pediatr Res.2009; 65(2): 249-51.
- Caffarini M, Armeni T, Pellegrino P, Cianfruglia L, Martino M, Offidani A, et al. Cushing Syndrome: The Role of MSCs in Wound Healing, Immunosuppression, Comorbidities, and Antioxidant Imbalance. Front Cell Dev Biol. 2019; 7: 227.
- opinion Ac. Obesity in Adolescents. The American College of Obstetricians and Gynecologists. 2020.
- Swallen KC, Reither EN, Haas SA, Meier AM. Overweight, obesity, and health-related quality of life among adolescents: the National Longitudinal Study of Adolescent Health. Pediatrics. 2005; 115(2): 340-7.
- Gortmaker SL, Must A, Perrin JM, Sobol AM, Dietz WH. Social and economic consequences of overweight in adolescence and young adulthood. N Engl J Med. 1993; 329(14): 1008-12.