Case Report - Volume 2 - Issue 5
Acute transverse myelitis after spinal anesthesia: Should anesthesia be condemned?
Richmond R Gomes
Associate Professor, Medicine, Ad-din Women’s Medical College Hospital, Bangladesh.
Received Date : Aug 22, 2022
Accepted Date : Sep 26, 2022
Published Date: Oct 05, 2022
Copyright:© Richmond Ronald Gomes 2022
*Corresponding Author : Richmond Ronald Gomes, Associate Professor,Medicine Ad-din Women’s Medical College Hospital, Dhaka Bangladesh.Tel: 8801819289499
Email:rrichi.dmc.k56@gmail.com
DOI: Doi.org/10.55920/2771-019X/1258
Abstract
Spinal anesthesia is widely used during surgical procedures. It is generally safe and the frequency of severe, permanent neurological complications associated with it has been reported to be extremely low. We report a patient, who developed paraplegia following spinal anesthesia. A 23-year-old lady developed acute transverse myelitis (ATM) with a rapid progression of acute motor sensory spastic paraplegia and autonomic dysfunction 24 hours after delivery of her first child by caesarean section. Spinal magnetic resonance imaging revealed myelitis at D9-12. She was given 1 gram methyl prednisolone daily for 5 days followed by oral prednisolone 1mg/kg/day which was tapered off in next 3 months. The neurological recovery was fairly good and the patient returned to full time work in 6 months. Since spinal anesthesia had been used in our case, a causal relationship can be assumed. This case emphasizes the danger of attributing all cases of transverse myelitis which have a close temporal relationship to spinal or epidural anesthesia, to the anesthetic technique itself.
Keywords: Spinal anesthesia; acute transverse myelitis; spastic paraplegia; methyl prednisolone.
Introduction
Spinal and epidural injections for anesthesia and analgesia has been a popular choice and one of the most cost effective means of providing anesthesia and analgesia during caesarean section . They are generally regarded as safe and the frequency of severe, permanent neurological complications related to them, based on prospective and retrospective studies, seems to be extremely low (roughly less than 0.01%). However, A wide array of temporary and permanent neurological complications has been reported with spinal anesthesia which includes severe spinal cord injury, including direct cord damage, epidural hematoma/abscess and neurotoxicity resulting from local anesthetics, cauda-equina syndrome, sciatic nerve palsy, transient paraplegia, quadriplegia, brain damage and even death [1,2]. This injury can result from direct trauma by needle, toxicity of anesthetic agent, epidural hematoma and ischemia from arterial injury or severe hypotension [2,3]. Any neurological deficits in the postoperative period should be immediately evaluated for early management. We present here a case of acute transverse myelitis (ATM) which manifested 24 hours after delivery by caesarean section surgery. It is important for anesthesiologist, surgeon and neurologist to be aware of transverse myelitis as a complication of spinal anesthesia. This case also brings up the difficulty encountered in determination of the interspace used for spinal anesthesia and the potential for traumatic injury to the spinal cord.
Case report
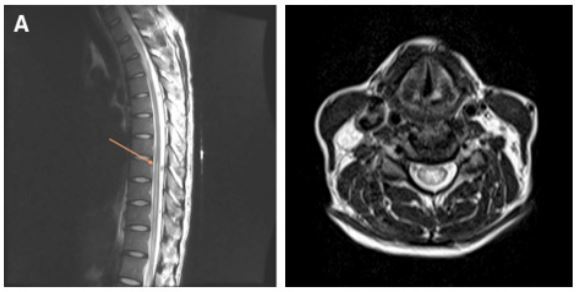
A 23 year old primi at her 38 weeks of gestation was admitted for caesarean section. The patient had an average built. His height was 148cm and weight 65 Kg. The patient gave a history of malaise without fever two weeks back, which resolved without any medication in 3-4 days. There was no significant medical history or medication noted. There was no past history of surgery or anesthesia. On examination, there was no significant clinical finding. Preoperative pulse rate was 82 beats/min , regular, good volume and blood pressure was 120/70 mm of Hg. Tablet Bromazepam 3 mg was given on previous night as premedication. Spinal anesthesia was planned for the surgery. The patient was preloaded with 1 litre of Lactated Ringer solution. After observing strict aseptic precautions, subarachnoid block was performed using a 24 gauge spinal needle (Quincke-Babcock spinal needle) at the L2-3 interspace using the midline approach, and 3.0 ml of 0.5% hyperbaric bupivacaine was administered. The effect was adequate. The intra operative period was uneventful. ECG showed normal sinus rhythm and the systolic blood pressure remained within the range of 110-120 mm Hg. Surgery lasted for about 30 minutes. Postoperative period was uneventful. The patient recovered completely from the effect of spinal anesthesia in the next 3 hours. On the first postoperative day about 20 hours after the surgery, the patient complained of severe, painful, electric shock-like sensation in both lower limbs, which was bilaterally symmetrical and paresthesia in the legs followed by ascending numbness upto the level of umbilicus and progressive weakness in both the legs. This was associated with fecal and urinary incontinence. On examination, the patient was afebrile and his vitals were stable. Neurological examination revealed spastic paraplegia (muscle power 2/5 in both lower limbs) with deficit of all sensory modalities till T9. There was sustained ankle clonus and Babinski responses bilaterally (extensor plantar). There was no neck stiffness. Fundoscopy, upper limbs, neck examination revealed no abnormalities. Findings on hematological and blood chemistry determinations were normal. Screening tests for syphilis (TPHA), autoantibodies (ANA, ANCA) and bacterial infection (Blood and urine culture) were normal. The cerebrospinal fluid examination revealed one abnormality-protein count was elevated to 95 mg% (normal 15-45 mg%). Chest X-ray was normal. MRI of dorso lumbar spine revealed Demyelination extending from D9 to D12 segment (Figure 1 and 2).
Figure 1 and figure 2: MRI of dorso lumbar spine showing Demyelination extending from D9 to D12 segment of spinal cord and axial section showing Demyelination respectively.
A diagnosis of acute transverse myelitis was made and the patient was started on Injection Methyl prednisolone 1 gm dissolved in 500 ml of 0.9% saline given intravenously over 2 hours; for a total of 5 days. This was followed by oral prednisolone 1 mg/kg/day from 6th day. Regular physiotherapy and prophylactic dose of low molecular weight heparin was started. She was discharged on 10th post operative day. There is a plan to taper the dose of steroid in next 3 months.
Neurological recovery was good. After 4 weeks the patient could stand independently and had regained normal sphincter control. Only after 6 months the patient recovered completely and returned to full time work. Hence, a relatively good recovery was achieved after a rather severe initial deficit
Discussion
Acute paraplegia in the early postoperative period is a challenging and potentially devastating anesthetic complication, which can occur when neuraxial techniques are used. Several clinical differential diagnoses are possible. Emergent MRI in this patient excluded cord compression by lesions such as herniated intervertebral discs, dislocated vertebral body or bony material, tumor, epidural abscess or epidural hematoma. According to the topographical distribution of neurological deficits, a thoracic intramedullary hyperintensity lesion (between T10 and T12) on the T2 weighted MRI scan and elevated protein in the CSF analysis, the diagnosis of thoracic ATM was established [4].
ATM is a rare clinical syndrome with an incidence of one to four patients per million, per year [5]. It is characterized by acute inflammation affecting the white and gray matter in multiple spinal cord segments, and causes rapid development of neurological deficits in motor, sensory, and autonomic functions. Motor weakness typically occurs initially in the lower legs and ascends to the waist rapidly, although bilateral arms may be involved occasionally. Loss of sensation in all modalities below a fixed level usually can be documented. Autonomic dysfunction varies, however, including increased urinary urgency, bowel or bladder incontinence, difficulty or inability to void, incomplete evacuation, or bowel constipation.6 Mostly, these neurological deficits progress to their nadir within hours or days [4]. The most common level in adults is the mid-thoracic region, whereas children have a higher frequency of involvement of cervical levels [7]. Based on expert opinion of the Transverse Myelitis Consortium Working Group, causes of ATM can be classified as either idiopathic or disease-associated ATM. The latter is usually a part of manifestations of systemic autoimmune disease, such as systemic lupus erythematous, multiple sclerosis, Sjogren syndrome, or neurosarcoidosis [8]. The remaining idiopathic ATM is difficult to find the definite causes but is generally thought as a parainfectious or autoimmune-based neuroinflammatory process [4]. Nearly half of the patients diagnosed with idiopathic ATM have a preceding febrile event such as respiratory, gastrointestinal, or systemic illness [9,10].
In the majority of cases of acute transverse myelitis the aetiology is unknown; preceding infections, mainly viral account for 27-37% of cases [11,12]. Syphilitic endarteritis was probably a common cause of acute transverse myelitis at the beginning of the century [13]. At present, vascular causes resulting mainly from atherosclerosis and collagen diseases are probably more common than syphilis [14,15].
Berman et al [12] developed a criterion for the diagnosis of Acute transverse myelitis which included:
- Acutely developing paraparesis affecting motor and sensory system as well as sphincters.
- Spinal segmental level of sensory disturbance (patients with patchy sensory deficit or Brown
Sequard syndrome were excluded).
- Stable, non progressive clinical course.
- No clinical or laboratory evidence of spinal cord compression and
- Absence of other known neurological disease, including malignant disease with metastasis, severe
back trauma and encephalitis. Patients with irradiation of the spine were also excluded.
In a study conducted by Berman et al [12], 3 patients gave history of unusual physical strain before the onset of acute transverse myelitis and 1 occurred after obstetric delivery. 8 patients had malaise without fever 3 days to 3 weeks before the onset of acute transverse myelitis.
The temporal relationship and proximity of an spinal needle for TEA to the affected regions of spinal cord easily raises the concern that spinal anesthesia or local anesthetic neurotoxicity may be the cause of the neurological deficits [16]. Although sporadic cases of ATM have been described following spinal [17] or epidural [18,19] anesthesia and even general anesthesia without neuraxial invasion, [20] none of them suggested a direct causal relationship after cautiously ruling out vascular and compressive etiologies of cord injury. Direct cord trauma either by needle was hardly possible in our patient since the needle was inserted while she was fully conscious. Once the cord was inadvertently penetrated or injected with local anesthetic, she should be aware of the injury and reported his suffering at the first moment. Importantly, the patient was doing well after surgery with freely movable extremities until acute deterioration of spinal cord function on POD 1. Excessive sensory and motor blockade from epidural local anesthetics was excluded since the sensory and motor deficits were not reversed after removal of spinal needle. Therefore, the progression of sensory, motor, and autonomic deficits, along with development of characteristic inflammatory spinal cord lesion determined by an MRI scan, strongly support the diagnosis of ATM. [4,17,18,19,20].
The causes of ATM in this patient in the early post- operative period may be secondary to an occult infection or by an immune-mediated neuroinflammatory mechanism. Since the autoimmune profile was normal, negative bacterial cultures may be explained by the aggressive use of postoperative antibiotics. Although high dose intravenous corticosteroid treatment to this situation is usually anecdotal, it was reasonable in this patient who yielded partial improvement in motor function8. In spite of failure of identification of an infectious source, the cord lesion was evidently neuroinflammatory in etiology, which explained the effect of high-dose steroid therapy.
Lack of information regarding temporal sequence of needle trauma, injection of lignocaine and paresthesias limits the ability to ascertain the etiology of such profound neurological deficit. The severe pain and the electric shock-like sensation passing symmetrically through both lower limbs immediately after insertion of needle, during the procedure were highly suggestive of direct cord injury by the needle. Paresthesias associated with spinal cord injury can occur at the time of needle placement but has also been reported at the time of injection or secondary to irritation, edema or hematoma [21, 22].
The assessment of the lumbar intervertebral space by palpation of anatomical landmarks for lumbar puncture can be grossly wrong, which explains the rather rostral lesion in our patient. Surveys have shown that the correct position was often not clearly identified and errors can range from one to several spaces above the presumed level of puncture site [23]. Similar discordance has been observed between actual level of puncture site and those recorded in operative notes [23,24]. Some such accidents occur when this is tried under sedation and the patient is unable to react to the initial pain on touching the nerve root of the spinal cord but cases have also been reported where paraplegia occurred during injection in an awake patient as evident in our case [24,25]. Pain is more common and severe in extra-axial lesions affecting the nerve roots or blood vessels that are innervated by sensory neurons mediating pain. Surprisingly, some patients do not experience any pain during the puncture of spinal cord and pass unnoticed even if the procedure is done under fluoroscopy [23,24].
In addition to direct needle trauma, neurotoxicity of the anesthetic agent or arterial occlusion or hypoperfusion could have contributed to paraplegia in this patient, since cytotoxicity of local and regional anesthesia is well established [26] This patient had paresthesias immediately after needle had traumatized the spinal cord and the injection of anesthetic agent most likely worsened the damage resulting in such a dense paraplegia.
The possible value of high-dose steroid treatment in this setting is unknown but given the poor prognosis, treatment by MPS should be considered. If used within eight hours, it has been shown to improve the outcome in other forms of injury [22].
Investigations to date strongly support that acute transverse myelitis is an autoimmune disease, lending support to studies which suggests that immunosuppressive therapy is beneficial [27]. In our case, acute transverse myelitis developed on the first postoperative day after a complete and normal recovery from the effect of spinal anesthesia. Also, the patient gave a history of malaise without fever 2 weeks prior to surgery.
Neurological complications following general anesthesia are not unknown [28,29]. Earlier studies and case reports have shown that transverse myelitis may follow even after an uncomplicated general anaesthesia [21]. Therefore, it appears that it is not always true to assume a close temporal relationship between regional anesthesia and transverse myelitis, since the aetiology remains unknown in majority of the cases.
Conclusion
In summary, this report describes the presentation and course of a patient with ATM in the early postoperative period spinal anesthesia. Using MRI and CSF analysis, ATM was established based on the neuroinflammatory origin of spinal cord injury. For early differential diagnoses and prompt management to improve outcomes of this rare and devastating complication, awareness of the potential complications of spinal anesthesia, continuous evaluation of neurological function after surgery and early MRI examination, if indicated, are warranted.
Conflict of interest: None Declared.
References
- Tripathi M, Nath SS, Gupta RK. Paraplegia after intra cord injection during attempted epidural steroid injection in an awake patient. Anesth Analg 2005; 101: 1209-11.
- Houten JK, Errico TJ. Paraplegia after lumbosacral nerve root block: Report of three cases. Spine J 2002; 2: 70-5.
- Tsui BC, Armstrong K. Can direct spinal cord injury occur without paresthesia? A report of delayed spinal cord injury after epidural placement in an awake patient. Anesth Analg 2005; 101: 1212-4.
- Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002; 59: 499e505.
- Berman M, Feldman S, Alter M, Zilber N, Kahana E. Acute transverse myelitis: incidence and etiologic considerations. Neurology 1981; 31: 966e71.
- Sakakibara R, Hattori T, Yasuda K, Yamanishi T. Micturition disturbance in acute transverse myelitis. Spinal Cord 1996; 34: 481e5.
- Pidcock FS, Krishnan C, Crawford TO, Salorio CF, Trovato M, Kerr DA. Acute transverse myelitis in childhood: center-based analysis of 47 cases. Neurology 2007; 68: 1474e80.
- Kaplin AI, Krishnan C, Deshpande DM, Pardo CA, Kerr DA. Diagnosis and management of acute myelopathies. Neurologist 2005; 11: 2e18.
- Jeffery DR, Mandler RN, Davis LE. Transverse myelitis. Retrospective analysis of 33 cases, with differentiation of cases associated with multiple sclerosis and parainfectious events. Arch Neurol 1993; 50: 532e5.
- Christensen PB, Wermuth L, Hinge HH, Bomers K. Clinical course and long-term prognosis of acute transverse myelopathy. Acta Neurol Scand 1990; 81: 431e5.
- Altrocchi PH: Acute transverse myelopathy. Archives of Neurology 1963; 9: 111-119.
- Berman M, Feldman S, Alter M, Zilber N, Kahane E : Acute transverse myelitis : incidence and etiologic considerations. Neurology 1981; 31: 966-971.
- Singer HD: Pathology of so called acute myelitis. Brain 1902; 25: 332-340.
- Granger DP: Transverse myelitis with recovery: the only manifestation with systemic lupus erythematosus. Neurology 1963; 9: 17-25.
- Weisman AD, Adams RD: The neurologic complications of dissecting aortic aneurysm. Brain 1944; 67: 69-92.
- Hindman BJ, Palecek JP, Posner KL, Traynelis VC, Lee LA, Sawin PD, et al. Cervical spinal cord, root, and bony spine injuries: a closed claims analysis. Anesthesiology 2011; 114: 782e95.
- Jha S, Kumar R. Transverse myelitis following spinal anesthesia. Neurol India 2006; 54: 425e7.
- Martinez-Garcia E, Pelaez E, Roman JC, Perez-Gallardo A. Transverse myelitis following general and epidural anaesthesia in a paediatric patient. Anaesthesia 2005; 60: 921e3.
- Lucas DN, Kennedy A, Dob DP. Dural puncture and iatrogenic pneumocephalus with subsequent transverse myelitis in a parturient. Can J Anaesth 2000; 47: 1103e6.
- Gutowski NJ, Davies AO. Transverse myelitis following general anaesthesia. Anaesthesia 1993; 48: 44e5.
- Kao MC, Tsai SK, Tsou MY, Lee HK, Guo WY, Hu JS. Paraplegia after delayed detection of inadvertent spinal cord injury during thoracic epidural in an anesthetized elderly patient. Anesth Analg 2004; 99: 580-3.
- Furman MB, Giovanniello MT, O’Brien EM. Incidence of intravascular penetration in transforaminal cervical epidural steroid injections. Spine 2003; 28: 21-5.
- Reynolds F. Damage to the conus medullaris following spinal anaesthesia. Anaesthesia 2001; 56: 238-47.
- Horlocker TT, Abel MD, Messick JM Jr, Schroeder DR. Small risk of serious neurologic complications related to lumbar epidural catheter placement in anesthetized patients. Anesth Analg 2003; 96: 1547-52
- Bulow PM, Biering-Sorensen F. Paraplegia, a severe complication to epidural analgesia. Acta Anaesthesiol Scand 1999; 43: 233-5.
- Johnson ME. Neurotoxicity of lidocaine: implications for spinal anesthesia and neuroprotection. J Neurosurg Anesthesiol 2004; 16: 80-3
- William R, Tyor. Postinfectious Encephalomyelitis and Transverse Myelitis. In: Johnson, Griffin. Editors. Current Therapy in Neurologic disease. Fourth Edition 155-157.
- Elizabeth J, Lipson SF, Bromage PR, Camporise EM: Neurologic complications following general anaesthesia. Anaesthesia 1983; 38: 226-229.
- Sinclair RN: Ascending spinal paralysis following hysterectomy under general anaesthesia. Anaesthesia 1954; 4: 286-287.