Case Report - Volume 2 - Issue 5
Neurological Complications of Infective Endocarditis: Beware Of Intracerebral Mycotic Aneurysms, Case Report
Moudafia Z1*; Lamri.H1; Dahmani B1; Omari Tadlaoui. S1; Alaoui Rachidi S1,2
1Radiology Department, Tangier-Tetouan-Al-Hoceima University Hospital. Morocco.
2Faculty of Medicine and Pharmacy of Tangier, Abdelmalek Essaadi University, 90000 Tangier, Morocco.
Received Date : Aug 26, 2022
Accepted Date : Oct 04, 2022
Published Date: Oct 15, 2022
Copyright:© Moudafia Z 2022
*Corresponding Author : Moudafia Z, Radiology Department, TangierTetouan-Al-Hoceima University Hospital, (2) Faculty of Medicine and Pharmacy of Tangier, Abdelmalek Essaadi University, 90000 Tangier, Morocco
Email: zinmoud@gmail.com
DOI: Doi.org/10.55920/2771-019X/1266
Abstract
The modal branching pattern of the aortic arch in humans consists of three large vessels arising from the aorta, which are: the brachiocephalic arterial trunk, the left common carotid artery, and the left subclavian artery. Birth defects of the supra-aortic vessels are usually asymptomatic and incidental. We report two cases of this variant of the normal.
Keywords: Infective endocarditis; Neurological complications Mycotic aneurysms Magnetic resonance imaging.
Introduction
Endocarditis is an inflammation of the endocardium and its structures (valves), most often of infectious origin, described by William Osler in 1885. Non-infectious causes of endocarditis (autoimmune, cancerous) are exceptional. Infective endocarditis (IE) is not a uniform disease: its presentations are highly variable, depending on the initial clinical manifestations, pre-existing heart disease if any, the microorganism, the presence or absence of complications, and the patient's characteristics. Its diagnosis is often difficult. It must be easily evoked. It is based on a combination of clinical, microbiological and echocardiographic evidence (modified Duke criteria). Echocardiography is the first imaging test to be performed rapidly in case of suspected infective endocarditis. An extension workup to screen for possible embolic complications should be performed as soon as possible, essentially including a thoracoabdomino-pelvic CT scan with iodinated contrast injection supplemented́ with brain imaging (CT scan or magnetic resonance imaging [MRI]) [1]. Neurological complications of infective endocarditis are frequent and are often the revealing mode of the disease after the infectious syndrome, They can jeopardize the vital and functional neurological prognosis and allow to significantly modify the management.
The systematic search for mycotic aneurysms, particularly intracranial, should not be neglected. These are often asymptomatic and can be revealed suddenly by a serious neurological picture [2].
Case presentation
A 36 year old patient without any particular pathological history, without any cardiovascular risk factors, without any notion of rheumatic fever or recurrent angina presented to the emergency room for an acute stroke. The patient underwent a cerebral CT scan without injection of PDC, which showed a very hypo dense lesion in the left thalamic brain with an ischemic vascular appearance. This was in favor of a chronic deep sylvian AVCI.
The patient reported the notion of dyspnea of effort, and feverish peaks for a few months of night sweats all evolving in a context of alteration of the general state. The clinical examination revealed a fever of 38.6 8C, tachypnea, blood pressure (BP) of 95/50 mm Hg with a heart rate (HR) of 105 beats/min. On cardiovascular examination, there was a holo-systolic murmur at the mitral focus and a B2 burst at the pulmonary focus. The biological workup revealed a hyperleukocytosis of 13,500 elements/mm3 and a C-reactive protein (CRP) of 45 mg/L.
The chest radiograph was without abnormalities but the Trans thoracic echocardiography was in favor of an infective endocarditis with mitral localization complicated by a severe mitral leak. The contractility and function of the left ventricle were preserved. The right cavities were not dilated and there was no pericardial effusion. The cerebral angioscan found two well-limited intracerebral aneurysms located in the right temporal and left occipital regions, the largest measuring 5 mm in diameter in the right temporal region and associated with microaneurysms, four of which were calcified. T1 T2 FLAIR MRI in the 3 diffusion planes (eSWAV / 3DTOF / 3DT1C+ / axial T1 C+) showed the presence of a small left thalamic area with a T2 and FLAIR hyper signal, with a non-restrictive T1 hypo signal of sequelae vascular ischemic appearance.
Associated with Two peripheral parenchymal aneurysms right temporal and left occipital measuring 6.7mm and 4mm in diameter respectively. Multiple juxta-cortical hypo signals fronto-parietal occipital and cerebellar bilaterally related to calcified microbleeds or microaneurysms MRI confirmed the presence of two right temporal and left occipital mycotic aneurysms.
The diagnosis of infective endocarditis complicated by intracerebral mycotic aneurysms was retained and confirmed by all the clinical, biological, echocardiographic and scannographic arguments. The blood cultures had allowed the isolation of the germ: streptococcus. Under intravenous antibiotic therapy for 4 weeks, the clinical and biological evolution was favorable. The patient did not benefit from a surgical treatment because of the results of her imaging, she was put under active surveillance with a medical treatment based on antibiotic therapy.
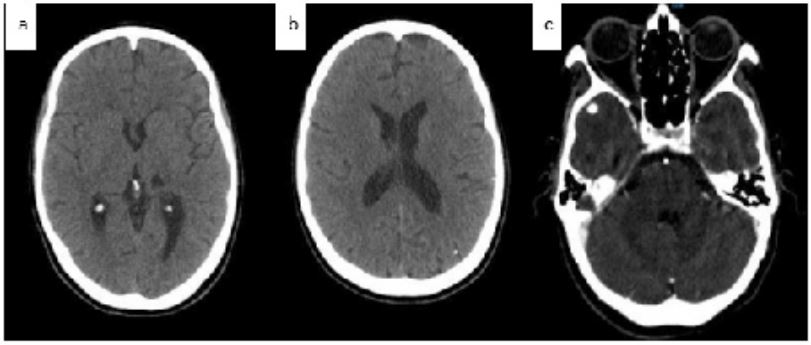
Figure 1: Axial CT scan showing hypo dense lesion in the left thalamic brain with an ischemic vascular appearance : chronic deep sylvian AVCI(a), small microcalcifications (b), cerebral angioscan showing well-limited intracerebral right temporal aneurysms (c).
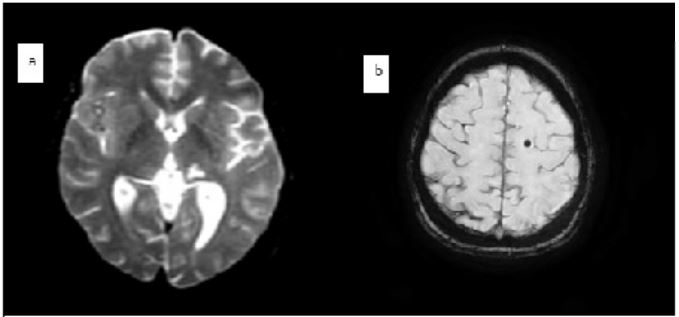
Figure 2: MRI showing small left thalamic area with a T2 hyper signal (a) juxta-cortical hypo signals in fronto-parietal : microbleeds (b)
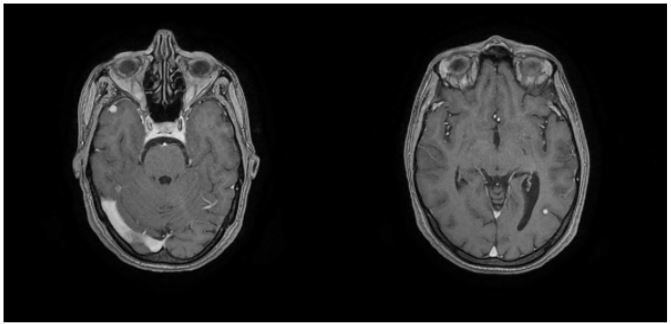
Figure 3: Angio-MRI showing two mycotic right temporal and left occipital aneurysms.
Discussion
Endocarditis is an inflammation of the endocardium and its structures (valves), most often of infectious origin, described by William Osler in 1885. Its presentations are very variable which makes its diagnosis often difficult. Indeed, it relies on blood cultures and echocardiography. Infective Endocarditis (IE) can present as an acute infection, with rapid progression, or as a subacutë or chronic disease with fever and nonspecific symptoms. Fever is often the telltale sign of the disease, but only when placed in context can its diagnostic value be clarified [3]. One of the main difficulties in the diagnosis of IE is that its initial symptoms are very variable from one patient to another [4]. In the case of our patient, the initial functional signs she presented were the notion of exertional dyspnea, and feverish peaks for several months of night sweats, all evolving in a context of alteration of the general state.
The demonstration of cardiac damage constitutes one of the major criteria for the diagnosis of IE. Echocardiography (transthoracic and transesophageal) remains the reference examination to determine the lesions and their impact. Cardiac CT and functional imaging (PET scan, labelled leukocyte scintigraphy) are also very interesting to authenticate cardiac damage, especially in the context of IE on intracardiac material [5,6].
In our patient, the cardiovascular examination revealed a holo-systolic murmur at the mitral site and a B2 burst at the pulmonary site. Trans thoracic echocardiography allowed us to diagnose infectious endocarditis with mitral vegetation complicated by a severe mitral leak. Infective endocarditis (IE) can affect almost all organs through its complications, and therefore its clinical manifestations are classically separated into cardiac and extracardiac complications through vascular and/or immunological phenomena. The infection starts initially in the heart with the formation of vegetation, which spreads locally and increases in size and number in the absence of treatment and will be responsible for a destruction of the valvular tissues then the infection spreads to the perivalvular space.
These anatomical lesions most often cause valvular insufficiency which leads to heart failure which is the most frequent complication of IE. This is the case of our patient who had a mitral insufficiency grafted on a pre-existing mitral stenosis, however his ventricular function was without abnormality. Neurological complications are the most feared and can be of embolic, hemorrhagic and/or infectious origin and are often at the origin of the diagnosis of IE: the parenchymal lesion will be suppurated, inflammatory or purely ischemic [7].
Cerebral MRI is the recommended examination for the detection of symptomatic and asymptomatic neurological complications [8]. Stroke is the most frequent neurological manifestation in IE: it is of ischemic or hemorrhagic origin. Cerebral embolisms, reported in 10 to 35% of cases, most often involve the middle cerebral artery territory. The presence of emboli of multiple locations, associated with fever, underlying heart disease at risk of IE, should raise suspicion of IE in a patient admitted for stroke. The diagnosis of an embolic event complicated by an IE contraindicates thrombolysis a priori, as it increases the risk of bleeding [9].
Our patient presented with a sudden onset stroke that led to a brain imaging that revealed a sequelae of ischemic thalamic plaques related to an old deep Sylvian stroke. Cerebral hemorrhages are rarer and represent 2 to 12% of neurological complications. They can be intracerebral or subarachnoid, and are related to three mechanisms: either a hemorrhagic transformation of an initial ischemic event, or to a rupture of an intracranial mycotic aneurysm or necrotizing pyogenic arteritis responsible for a rupture of an intracranial vessel. They significantly worsen the prognosis [10].
Cerebral MRI, via the T2* sequence, allows the detection of silent microscopic hemorrhagic lesions, "microbleeds", which are not visible on CT (11) . Like ischemic lesions, microbleeds could contribute to the diagnosis of IE (I. Klein et al., personal data).
In our patient's case, MRI also revealed multiple juxta-cortical hypo signals in the fronto-parietal, occipital and cerebellar regions bilaterally in connection with microbleeds or calcified micro-aneurysms.
Hemorrhagic transformation of a DVA may occur in a second stage (10-20% of cases), even in the absence of effective anticoagulation (12). It may be the leading cause of death from massive intracranial hemorrhage during AEs (13). The risk of hemorrhagic transformation is directly related to the size of the infarct [14].
Arterial lesions leading to intracranial hemorrhage are either infectious necrotizing arteritis or mycotic aneurysms, which may rupture immediately or several weeks after diagnosis. Intracranial hemorrhage due to necrotizing arteritis most often occurs in the setting of acute left-sided IE with Staphylococcus aureus [15,16]. Patients present with an acute febrile headache and neurologic deficit of abrupt onset, revealing a cerebral or subarachnoid hemorrhage. Other neurological manifestations include bacterial or non-bacterial meningitis (2-5% of cases) and true brain abscesses. More rarely, mental disorders (lethargy, disorientation, depression, confusion, etc.) are quite common in the elderly. Mycotic aneurysms or infective aneurysms are a classic but rare complication and represent less than 5% of IE complications and less than 10% of neurological complications when they involve intracranial vessels [17,18,19].
They result from septic embolism of vegetations either in the arterial vasa vasorum or in the arterial lumen, with extension of the infection by contiguity from inside to outside through the intima and the wall of the affected vessel. They affect, in order of frequency, the cerebral arteries, the visceral arteries, and finally the arteries of the limbs. They can be totally asymptomatic in 8% to 32% of cases, or be revealed in an acute manner by their rupture which becomes serious and of bad prognosis, in particular at the cerebral level. In fact, cerebral imaging, especially injected imaging, must systematically detect them, especially when a neurological sign appears, even a minor one, such as headache or meningeal stiffness.
Cerebral angioscan and cerebral MRI angiography are two diagnostic means that allow the positive diagnosis of intracranial mycotic aneurysms, with an identical sensitivitý if the size is greater than 5mm (approximately 95%). Angioscanner may be insufficient to detect small skull base aneurysms [20]. On imaging Intracranial mycotic aneurysms appear as a well-limited, irregular, fusiform vascular formation with an ill-defined aneurysmal neck. Their number is multiple in 25% of cases, and they are predominantly located distally, mainly at the level of the bifurcations of the middle cerebral artery [21,22].
Their indirect signs are visualized on CT or ideally on cerebral MRI, which can also orient their location [44]. These signs are either cerebral and/or subarachnoid hemorrhage, which indicate their rupture, and certain associated signs such as secondary ischemic parenchymal lesions, cerebral edema or even hydrocephalus.
In the case of our patient, both imaging methods were performed (cerebral angioscan and cerebral MRI) and allowed to highlight both direct and indirect signs: They confirmed the presence of right temporal and left occipital mycotic aneurysms visible on CT and MRI associated with deep sylvian sequential ischemic lesions and multiple juxta-cortical supratentorial and subtentorial hypo signals evoking microbleeds or calcified microaneurysms. Nevertheless, cerebral angiography remains the reference but invasive examination, especially for the diagnosis of small aneurysms [22]. The management of these asymptomatic aneurysms is not codified (23). Some of them will require a follow-up by imaging with favorable evolution under antibiotic therapy, others will benefit from a preventive management by endovascular embolization technique rather than by neurosurgery, and this in particular before cardiac surgery. It is also necessary to discuss whether or not to continue anticoagulant treatment, which can be a real dilemma in patients with prostheses.
Neurological complications may be embolic, hemorrhagic, and/or infectious in origin, and are often the mode of revelation of IE and are a poor prognostic factor. Embolic complications are very frequent, and can affect all organs: brain, spleen and kidney most often for left heart IE, lungs for right heart IE. They are related to the fragmentation and migration of vegetations and can affect all organs, and cerebral embolisms alone account for more than 50% of embolic accidents [24,25].
In the case of our patient, the injected examinations showed permeable and normo-opacified intracranial vessels. They can be asymptomatic and highlighted by imaging performed as a matter of course: cerebral MRI, cerebral CT scan that detect these emboli. The discovery of emboli at a distance often influences the indication for surgery as a means of preventing the risk of embolism (26). Therefore, European recommendations advise the use of complementary imaging examinations, in particular cerebral MRI, to detect them.This is the case of our patient in whom the surgical indication was influenced by the discovery of aneurysms and probable sequential ischemic and hemorrhagic lesions, she benefited from active monitoring and treatment with antibiotics.
Conclusions
Neurological complications of infective endocarditis are frequent and have a particular importance because they are often inaugural, can be life-threatening, and can significantly alter management. They must be looked for in front of any focal neurological sign or unexplained disorder of consciousnesś especially in a young subject. Ischemic strokes due to vegetation embolism are the main neurological complication of endocarditis. Mycotic aneurysms are infectious aneurysms and constitute a classic but rare complication that must be systematically detected by various non-invasive imaging means because they are in most cases asymptomatic and must be managed on an individual basis.Magnetic resonance imaging with injection remains the reference examination to detect these neurological complications.
References
- Endocardite infectueuse Dr Fabrice Camou1, Dr Marina Dijos2 1. Service de médecine interne et maladies infectieuses (Pr Pellegrin), hôpital Haut-Lévêque ; service de réanimation médicale, hôpital Saint-André, CHU de Bordeaux, France.
- R Sonnevillea, I Kleinb, L Bouadmaa, B Mourvilliera, B Regniera, M. WolffaComplications neurologiques des endocardites infectieuses Neurologic complications of infective endocarditis, a Service de réanimation médicale et infectieuse, hôpital Bichat—Claude-Bernard, université Paris 7, 46, rue Henri-Huchard, 75877 Paris cedex 18, France.
- b Service de radiologie, hôpital Bichat—Claude-Bernard, université Paris 7, 46, rue Henri-Huchard, 75877 Paris cedex 18, France Reçu le 4 juin 2009 ; accepté le 14 juin 2009 Disponible sur Internet le 3 aouˆt 2009.
- Diagnostic de l'endocardite infectieuse François Delahaye, Guy De Gevigney Université Claude-Bernard Lyon I, hôpital Louis-Pradel, hospices civils de Lyon, 69008 Lyon, France Correspondance : François Delahaye, Hôpital Louis-Pradel, 28, avenue du Doyen-Lépine, 69677 Bron cedex, France. francois.delahaye@chu-lyon.fr
- Prendergast BD. The changing face of infec tive endocarditis. Heart 2006; 92: 879–85.
- Lamas CC, Eykyn SJ. Bicuspid aortic valve: a silent danger: analysis of 50 cases of infective endocarditis. Clin Infect Dis 2000; 30: 336–41.
- Delahaye F, Goulet V, Lacassin F, Ecochard R, Selton-Suty C, Hoen B, et al. Characteristics of infective endocarditis in France in 1991. A 1- year survey. Eur Heart J 1995; 16: 394–401.
- Francioli P. Complications of infective endocarditis. In: Lippincott-Raven, editor. Infections of the central nervous sys- tem. Philadelphia; 2004; 523-53.
- Champey J, Pavese P, Bouvaist H, Kastler A, Krainik A, Francois P. Value of brain MRI in infective endocarditis: a narrative literature review. Eur J Clin Microbiol Infect Dis 2016; 35: 159–68.
- Asaithambi G, Adil MM, Qureshi AI. Throm- bolysis for ischemic stroke associated with infective endocarditis: results from the nationwide inpatient sample. Stroke 2013; 44: 2917–9.
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, Ivanova-Georgieva R, Noured- dine M, Plata A, et al. Neurological compli- cations of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127: 2272–84.
- Klein I, Iung B, Wolff M, Brochet E, Longuet P, Laissy JP, et al. Silent T2* cerebral microbleeds: a potential new imaging clue in infective endocarditis. Neurology 2007; 68: 2043.
- Cerebral Embolism Study Group. Cardioembolic stroke, early anticoagulation, and brain hemorrhage. Arch Intern Med 1987; 147: 636-40.
- Masuda J, Yutani C, Waki R, Ogata J, Kuriyama Y, Yamaguchi T. Histopathological analysis of the mechanisms of intracra- nial hemorrhage complicating infective endocarditis. Stroke 1992; 23: 843-50.
- Adams Jr HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Fur- lan A, et al. The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 2007; 115: e478-534.
- Le Cam B, Guivarch G, Boles JM, Garre M, Cartier F. Neurologic complications in a group of 86 bacterial endocarditis. Eur Heart J 1984; 5: 97-100.
- Weinstein L. Life-threatening complications of infective endocarditis and their management. Arch Intern Med 1986; 146: 953-7.
- Gillinov AM, Shah RV, Curtis WE, Stuart RS, Cameron DE, Baumgartner WA, et al. Valve replacement in patients with endocarditis and acute neurologic deficit. Ann Thorac Surg 1996; 61: 1125-9.
- Kannoth S, Thomas SV. Intracranial microbial aneurysm (infectious aneurysm): Current options for diagnosis and mana- gement. Neurocrit Care 2009; 11: 120-9.
- Peters PJ, Harrison T, Lennox JL. A dangerous dilemma: management of infectious intracranial aneurysms complicating endocarditis. Lancet Infect Dis 2006; 6: 742-8.
- White PM, Teasdale EM, Wardlaw JM, Easton V. Intracranial aneurysms: CT angiography and MR angiography for detec- tion prospective blinded comparison in a large patient cohort. Radiology 2001; 219: 739-49.
- Peters PJ, Harrison T, Lennox JL. A dangerous dilemma: management of infectious intracranial aneurysms complicating endocarditis. Lancet Infect Dis 2006; 6: 742-8.
- Baddour LM, Wilson WR, Bayer AS, Fowler Jr VG, Bolger AF, Levison ME, et al. Infective endocarditis: diagnosis, antimicro- bial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheu- matic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia. American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111: e394-404.
- Peters PJ, Harrison T, Lennox JL. A dangerous dilemma: management of infectious intracra- nial aneurysms complicating endocarditis. Lancet Infect Dis 2006; 6: 742–8.
- Duack DT, Lukes AS, Bright DK, the Duke Endocarditis Service. New criteria for diagnosis of infective endocarditis: utilization of spe cific echocardiographic findings. Am J Med 1994; 96: 200–9.
- Li JS, Sexton DJ, Mick N, Nettles R, Fowler Jr VG, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30: 633–8.
- HabibG,LancellottiP,AntunesMJ,Bongiorni MG, Casalta J-P, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Manage- ment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: Eur- opean Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36: 3075-128.

