Case Report - Volume 2 - Issue 5
Giant left atrium and Giant Eustachian valve in a patient with stenosis of mitral valve prosthesis
Marija Kotevska Angjushev1*; Darko Angjushev2 and Andrijan Kartalov2
1Department of Cardiac Surgery, Ss. Cyril and Methodius University in Skopje, North Macedonia.
2Department of Anaesthesiology, University Clinic for Traumatology, Orthopaedic Diseases, Anaesthesia, Reanimation, Intensive Care and Emergency Centre, Ss. Cyril and Methodius University in Skopje, North Macedonia.
Received Date : Aug 29, 2022
Accepted Date : Oct 05, 2022
Published Date: Oct 19, 2022
Copyright:© Marija Kotevska Angjushev 2022
*Corresponding Author : Marija Kotevska Angjushev, Department of Cardiac Surgery, Ss. Cyril and Methodius University in Skopje, North Macedonia
Email: angusevm@hotmail.com
DOI: Doi.org/10.55920/2771-019X/1268
Abstract
Giant left atrium (LA) is a extremely rare condition due to decline in rheumatic heart disease incidence. Giant Eustachian valve (EV) is an exceptionally rare finding, visualized on echocardiography in only 0.20% in adults. Typically is functionless in adults. We report a giant LA due to prosthetic mitral valve (MV) stenosis, seen on echocardiography, with late stage presentation in a 73-year-old woman. She had a history of MV replacement due to rheumatic MV disease 20-years ago. Echocardiography was suggestive for severe stenosis of MV prosthesis and giant LA with a size of 12.6x13.1cm, while computed tomography scan revealed a LA size of 17 × 11 cm. Giant EV was accidental finding on echocardiogram, that extended from the orifice of the inferior vena cava to the interatrial septum, without clinical significance. The patient was offered to undergo cardiac surgery on many occasions, but she kept refusing surgery. At last she accepted,unfortunately she died before proceeding to surgical intervention. A rheumatic giant LA due to stenosis of MV prosthesis is rarely reported entity in the literature and may be associated with significant morbidity and mortality. Optimal timing for surgery is crucial. Giant EV is rare accidental finding on echocardiography. Pre-operative identification of this structure is important, as it could be mistaken for pathology, ending with unnecessary treatment. This is a first echocardiographic report of coexistence of giant LA and giant EV.
Keywords: Giant left atrium; Giant Eustachian valve; Prosthetic mitral valve stenosis; Echocardiography; Rheumatic mitral valve disease.
Abbreviations
LA: Left Atrium; MV: Mitral Valve; EV: Eustachian Valve; AF: Atrial Fibrillation; TTE: Transthoracic Echocardiography; IVC: Inferior Vena Cava; RA: Right Atrium; CT: Computed Tomography (CT); LV: Left Ventricle; RV: Right Ventricle; MRI: Magnetic Resonance Imaging.
Introduction
Giant left atrium (LA) is a very rare entity and rarely reported in the literature due to decreased frequency of rheumatic mitral valve (MV) disease in the last four decades. Earlier diagnosis and treatment of rheumatic MV disease contributed to the considerable decline in the rheumatic heart disease incidence, especially in developed countries [1]. Giant LA is uncommonly seen in rheumatic mitral stenosis, but is usually related to severe mitral regurgitation or mixed mitral disease [2,3]. Concomitant atrial fibrillation (AF) is always present. Transthoracic echocardiography (TTE) is an excellent, most commonly used non-invasive method for diagnosis, follow up of MV disease and for estimating size of LA [4].
An Eustachian Valve (EV) is a remnant of embryonic valve of inferior vena cava (IVC). It is situated at the junction of IVC and inferior right atrium (RA) [5]. In adults it is usually absent or it may be present as a thin ridge on echocardiography. Sometimes, it may appear as a rigid, elongated, prominent structure, with huge range of variability from a thin ridge [5,6] to a giant structure projecting into RA cavity [7-10]. Giant EV is very rarely seen, in only 0.20% of adults, usually as a incidental finding on echocardiogram. In adults it is counted for a benign rudimentary structure and usually is asymptomatic. Typically does not have any pathologic significance in adults [6]. Identification of giant EV on pre-operative imaging is important because it could be mistaken for pathology, ending with a wrong diagnosis and unnecessary treatment [7-10].
Herein, we report a rarely reported entity of giant left atrium due to prosthetic mitral valve stenosis, presented in very late stage. Accidental finding on echocardiography is a giant Eustachian valve, which is rarely found, without any clinical significance for the patient
Case report
A 73-year-old woman was admitted to cardiac surgery department with clinical signs of congestive heart failure. She had a mechanical mitral Medtronic Hall prosthesis implanted 20 years ago for rheumatic MV disease and was in permanent AF ever since. Two years ago echocardiogram revealed severe stenosis of prosthetic mitral valve. On many occasions we recommended her a cardiac surgery, but she kept refusing and had been taking medical treatment. Since then, she had several recurrent admissions for congestive heart failure. At the present admission, the heart rate was 77/min and the blood pressure was 110/70 mmHg. Chest examination showed bilateral basal crepitation’s and peripheral edema were present. Heart examination revealed a sharp click of the prosthesis. The patient was on a continuous acenocoumarol therapy and had never developed thromboembolic complications. At the time of admission, she had higher international normalised (INR) ratio (7.0) than a recommended range. Chest X-ray demonstrated massive cardiomegaly (Figure 1). Abdominal ultrasound showed congestive hepatomegaly and a moderate volume of transudative ascites (video 1). TTE demonstrated severe calcified stenosis of the mitral valve prosthesis (area 0.9cm2, mean gradient 12 mmHg). A giant LA with dense whirling spontaneous echo contrast was present. Left atrium was 12.6 x 13.1cm in apical four chamber view (Figure 2,3 and video 2) and 9.5cm in parasternal long axis view. Using the ellipsoid formula the left atrial volume was calculated to be 820ml. There was no evidence of thrombus in the LA. A linear membranous structure was found in the RA cavity (Figure 4,5 and video 3). This structure originated from the orifice of IVC and extended into inferior interatrial septum and was diagnosed to be a giant Eustachian valve. The left ventricle (LV) was small (ejection fraction 40%) with severe diastolic dysfunction (Figure 2,3 and video 2). Severe pulmonary hypertension (estimated at 90mmHg) was present. The IVC and hepatic veins were dilated. Computed tomography (CT) scan was done to assess the relation of LA to surrounding structures. CT scan revealed a markedly dilated LA measuring 17×11cm, without compression of the adjacent organs. (Figure 6,7). No thrombi in the left atrial appendage and LA were present.
The patient was admitted for reoperation (replacement of mitral valve prosthesis and left atrial volume reduction surgery) as she finally accepted operation. During hospitalization her INR was corrected to therapeutic range of 2.5-3.5. We tried to stabilize her and optimize for operation, but unfortunately she died before proceeding to surgical intervention.
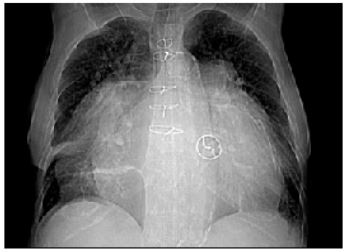
Figure 1: Chest X-ray shows massive cardiomegaly.
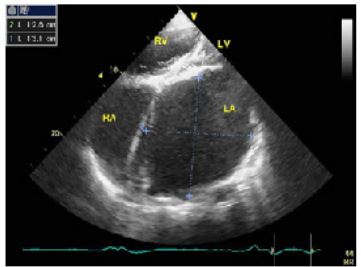
Figure 2: Apical four chamber view on transthoracic echocardiography. The LA was enormously dilated and anatomically distorted, thus it was difficult to make correct and adequate measurements of LA.
LA:left atrium; LV:left ventricle; RA:right atrium; RV:right ventricle;
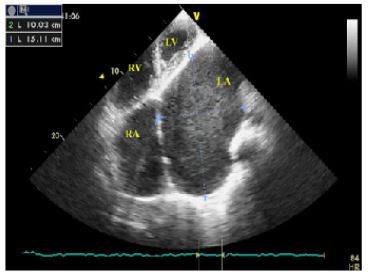
Figure 3: Apical four chamber view on transthoracic echocardiography demonstrates giant left atrium with dense spontaneous echo contrast, compressing the lateral wall of LV.
LA:left atrium; LV:left ventricle; RA: right atrium; RV: right ventricle;
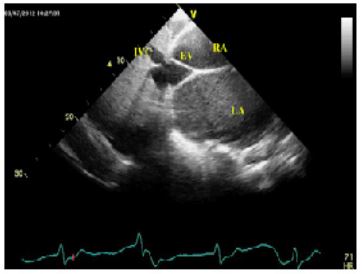
Figure 4: Subxiphoid view on transthoracic echocardiography demonstrates a giant EV extending from anterior rim of IVC orifice to inferior portion of interatrial septum. Its laminar dense structure and its origin from ostium of IVC are pathognomic for EV [8,23,25]. The subxiphoid view is diagnostic because both structures (EV and IVC) can be visualized at same time and at same imaging view [24]. The EV was rigid and was not observed obstruction to flow at the level of EV.
EV:Eustachian valve; IVC: inferior vena cava; RA:right atrium; LA:left atrium;
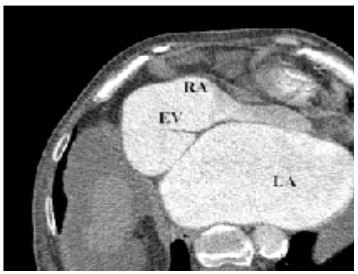
Figure 5: CT scan demonstrates a giant EV with insertion point into lower interatrial septum.
EV:Eustachian valve; RA:right atrium; LA:left atrium;
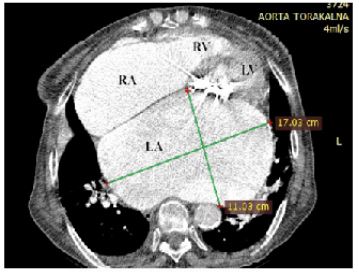
Figure 6: Contrast computed tomography scan-axial view revealed a giant left atrium measuring 17 × 11 cm, finding no thrombus in LA and left atrial appendage. Prosthetic mitral valve calcification is clearly seen.
RA: right atrium; RV: right ventricle; LA:left atrium; LV:left ventricle;
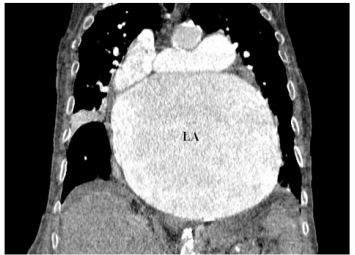
Figure 7: CT scan in coronal view demonstrating giant LA.
LA:left atrium;
Video 1. Subcostal view on transthoracic echocardiography is showing giant left atrium and moderate volume of perihepatic ascites.
Video 2. Apical four chamber and parasternal short axis view on transthoracic echocardiography showing a giant left atrium compressing the lateral wall of the left ventricle. Spontaneous echo contrast in LA cavity is visible.
Video 3. Subcostal view on transthoracic echocardiography is showing enlarged left atrium and giant Eustachian valve.
Discussion
Giant LA is a rarely seen in clinical practice, usually associated with rheumatic mitral valve disease [11]. Giant LA is uncommonly related with severe mitral stenosis [12], but most often is related with mitral regurgitation or mixed mitral disease [2]. Giant LA is defined by Kothari as LA diameter more than 8cm [11]. Other investigators [3,13] have used decreased LA diameter (more than 6.5cm) for the definition of giant LA. It was first described in 1849 by Hewett in autopsy series of mitral valve disease [14]. The mechanism of formation of giant LA is proposed due to rheumatic pancarditis because of the strong relation with rheumatic heart disease. Rheumatic pancarditis damages whole heart, including both atria by causing chronic inflammation, fibrosis within the walls with consecutive weakening of its walls. In such conditions LA enlarges more easily [2,15,16]. Intracavitary chronic pressure overload and volume overload produced by mitral valve disease are additional factors for LA enlargement. AF secondary to LA enlargement is always present. AF causes further growth of LA size due to diffuse atrophy of atrial muscle [15]. Both factors, LA wall weakening produced by rheumatic pancarditis and pressure overload produced by stenosis of mitral prosthesis were involved in development of giant LA in our patient. Permanent AF additionally caused further LA enlargement. Complications of giant atria include thromboembolism, AF, hemodynamic, respiratory complications, intracardiac and extracardiac compression symptoms [2,15,17]. Increased LA pressure is transmitted retrograde into pulmonary veins and capillaries and in the end into pulmonary arteries with consecutive increased pulmonary pressure enough to produce pulmonary edema. Pulmonary hypertension leads to increased right ventricular systolic pressure and to right heart failure [18]. Cardiac output can be reduced due to compression of the posterior and lateral wall of LV by severely enlarged LA [17], as it was in our case. In our patient, congestive heart failure symptoms were present, while extra cardiac compressive symptoms or thromboembolic events were absent.
Initial diagnostic tools are imaging modalities. TTE is most feasible and best non-invasive method to establish diagnose, to monitor MV disease progression and to assess LA size [4]. Additional imaging modalities, such magnetic resonance imaging (MRI) or cardiac CT are reliable in providing a precise measurement of LA size and in assessing its relations with surrounding organs. They are also helpful in diagnosing a thrombus in left atrial appendage or LA cavity [19]. There are several reports of giant LA described before [2,15,16,20,21]. The largest reported LA size was 22.3x19.2x20.1cm on MRI in a 50-year-old female patient with rheumatic mitral valve disease [20]. In our case, the maximal diameter of LA was smaller compared to this case and was 17cm on CT scan.
Role of medical therapy is limited to antiarrhythmics, anticoagulation and heart failure therapy. Management of most patients with giant LA is surgery, presented by mitral valve replacement with or without LA volume reduction [17]. Presence of a giant LA significantly increases mortality rates in mitral valve surgery, from 7% to 20% [22]. Delaying mitral valve replacement surgery may lead to deadly outcome. When surgery is delayed, elevated pulmonary pressure leads to to elevation in right heart pressure and this critically affects mortality rates [16]. Optimal timing is of vital importance. We advised our patient to undergo cardiac surgery on numerous occasions, but she has been continuously refusing operation.
The EV is an embryologic remnant of IVC valve, luying at junction of IVC and inferior RA. In fetal heart directs oxygenated blood from IVC towards foramen ovale and left atrium into systemic circulation. After birth, following closure of foramen ovale, EV has no particular function in adults and disappears completely in most of the cases. Sometimes, on echocardiographic examinations the EV may appear as a leaflike linear structure from orifice of IVC [23]. However, prominent EV is rarely seen on echocardiography with different presentations in shape, thickness and size [7]. Prominent EV is defined as a prominence over 1 cm into the RA [23]. Our patient had very elongated (at least 6 cm) giant EV extending from IVC to inferior portion of the interatrial septum, which seemed on echocardiography as a septal structure dividing the cavity of RA into two chambers. Such echocardiographic appearance of a divided RA cavity by giant EV has been reported before in very few case reports [7-9]. The septation created by the giant EV is generally incomplete, thus this condition is hemodynamically insignificant in most adults, as it was in our case [24]. Diagnostic pitfalls with EV variants mimicking pathology on echocardiography are often reported in the medical literature. Knowledge about anatomic variants of EV ensures accurate interpretation of echocardiographic findings avoiding misinterpretation from pathology. On pre-operative echocardiography, giant EV should be accurately identified as benign structure, especially should be differentiated from cor triatriatum dexter in order to avoid unnecessary surgical correction [7-10,23-25].
In summary, giant LA is extremely rarely observed in present era. Generally it is due to severe rheumatic mitral valve disease. TTE is accurate noninvasive method to diagnose and monitor the mitral valve disease progression and to evaluate left atrial size. Surgery is the mainstay of treatment, optimal timing can be life saving. Giant EV in adults is incidentally diagnosed by cardiac imaging techniques, usually without clinical consequences, but it is important to be recognized as benign structure on preoperative imaging. As far as we know, this seems to be a first echocardiographic report of a patient with giant LA and giant EV.
Acknowledgements: None.
References
- Fattouch K, Sampognaro R, Coppola G et al. Giant left atrium: a condition that is rarely seen today. J Cardiovasc Med (Hagerstown). 2008 Sep; 9(9): 967-8.
- Funk M, Perez M, Santana O. Asymptomatic giant left atrium. Clin Cardiol. 2010 Jun; 33(6): E104-5.
- Hurst W: Memories of patients with a giant left atrium. Circulation 2001; 104: 2630–2631.
- Hirata T, Wolfe SB, Popp RL, et al. Estimation of left atrial size using ultrasound. Am Heart J. 1969 Jul; 78(1): 43-52.
- Allen HD, Gutgesell HP, Clark EB, et al. Heart disease in infants, children and adolescents. Vol. 2, 6th ed. Philadelphia, PA:Lippincott Williams and Wilkins; 2001; 794-6.
- Schuchlenz HW, Saurer G, Weihs W, et al. Persisting eustachian valve in adults: relation to patent foramen ovale and cerebrovascular events. J Am Soc Echocardiogr. 2004 Mar; 17(3): 231-3.
- Yavuz T, Nazli C, Kinay O et al: Giant eustachian valve: with echocardiographic appearance of divided right atrium. Tex Heart Inst J. 2002; 29: 336-8.
- Oyedeji AT, Akintunde AA, Ajayi EA, et al. Coexistence of Cor triatriatum sinistrum and a prominent Eustachian valve mimicking a Cor triatriatum dextrum. J Cardiovasc Dis Res. 2012; 3(2): 170-172.
- Ozeke O, Celik A, Basel MC. Cor triatriatum dexter and sinister appearance caused by giant eustachian valve and prominent interatrial septal aneurysm. J Am Soc Echocardiogr. 2008 Jan;21(1): 91.e1-2.
- Angjushev D, Kotevska-Angjushev M, Lazarevski M. A giant eustachian valve protruding into the right ventricle: A case report. Archives of Cardiovascular Imaging 2. 2014 May; 3: 3.
- Kothari AA, Kothari K A. A giant left atrium. J Postgrad Med, 2005; 51: 49-50.
- Agrawal AK, Gupta AK, Gupta VK, et al. Giant Left Atrium: A Rare Entity in Rheumatic Valvular Heart Disease. Journal, Indian Academy of Clinical Medicine 2008; 9(3): 227-229.
- Kawazoe K, Beppu S, Takahara Y, et al. Surgical treatment of giant left atrium combined with mitral valvular disease. Plication procedure for reduction of compression to the left ventricle,bronchus and pulmonary parenchyma. J Thorac Cardiovasc Surg.1983; 85: 885–892.
- Hewett P. Aneurysmal dilation of the left auricle with thickening and contraction of the left ventricular opening. Trans Pathol Soc London. 1849/1850: 2–193.
- Seyfeli E, Akoglu S, Karazincir S, et al. Giant left atrium mimicking a right thoracic mass: case report. J Am Geriatr Soc. 2006 Jan; 54(1): 183-4.
- Ates M, Sensoz Y, Abay G, et al. Giant left atrium with rheumatic mitral stenosis. Tex Heart Inst J. 2006; 33(3): 389-91.
- Apostolakis E, Shuhaiber JH. The surgical management of giant left atrium. Eur J Cardiothorac Surg. 2008; 33: 182–190.
- Chick JF, Sheehan SE, Miller JD,et al. Giant left atrium in rheumatic heart disease: the classic signs of left atrial enlargement. J Emerg Med 2013; 44(6): e393–4.
- Root JD, Hammerman AM, Fischer KC. The CT appearance of the giant left atrium. Clin Imaging. 1990 Oct-Dec; 14(4): 305-8.
- Pandit BN, Aggarwal P, Subramaniyan S, et al. Largest giant left atrium in rheumatic heart disease. J Cardiol Cases. 2020 Dec 24; 24(1): 10-13.
- Goldenberg G, Eisen A, Weisenberg N, et al. Giant Left Atrium. The Israel Medical Association journal, 2009 Oct; 11(10): 641.
- Pomerantzeff MA. Tangential Triangular Resection for Correction of Giant Left Atrium. ISMICS (Annual Scientific Meeting-Invitation, Technologies and Techniques in Cardiothoracic and Cardiovascular /Vascular Surgery ). 2013 June 12-15.
- Moral S, Ballesteros E, Huguet M, Panaro A, Palet J, Evangelista A. Differential Diagnosis and Clinical Implications of Remnants of the Right Valve of the Sinus Venosus. J Am Soc Echocardiogr. 2016 Mar; 29(3): 183-94.
- Kim MJ, Jung HO. Anatomic variants mimicking pathology on echocardiography: differential diagnosis. J Cardiovasc Ultrasound. 2013; 21(3): 103-112.
- Limacher MC, Gutgesell HP, Vick GW, et al. Echocardiographic anatomy of the Eustachian valve. Am J Cardiol 1986; 57: 363-5.

