Case Report - Volume 2 - Issue 5
Pachydermoperiostosis (Touraine–Solente–Gole syndrome) imitating Acromegaly: A Rare Case Report
Richmond Ronald Gomes
Associate Professor, Department of Medicine, Ad-din Women’s Medical College Hospital, Dhaka, Bangladesh.
Received Date : Aug 26, 2022
Accepted Date : Oct 10, 2022
Published Date: Oct 27, 2022
Copyright:© Richmond Ronald Gomes 2022
*Corresponding Author : Richmond Ronald Gomes, Associate Professor, Department of MedicineAd-din Women’s Medical College Hospital, Dhaka, Bangladesh.
Email: rrichi.dmc.k56@gmail.com
DOI: Doi.org/10.55920/2771-019X/1275
Abstract
Pachydermoperiostosis (PDP), also known as Touraine-Solente-Golé syndrome/Rosenfeld-Kloepfer syndrome/primary or idiopathic Hypertrophic osteoarthropathy, is an autosomal-dominant/autosomal recessive inherited disorder with variable expression. In its complete form, it is characterized by pachyderma (thickening of the facial skin), skeletal changes (periostosis), excessive sweating (hyperhydrosis), and acropachia (digital clubbing) and presents usually at puberty. At least two gene mutations have been implicated, namely 15-hydroxyprostaglandin dehydrogenase (15HPGD) and SLCO2A1. Clinical manifestations of PDP are thought to relate to excessive collagen formation and dysregulation of matrix proteins because of fibroblastic hyperactivation. Disease progresses for 5–20 years before stabilizing. We describe a case of 25 year old male who presented with thickened skin on the face and scalp (resembling cutis verticis gyrata), palmoplantar hyperhidrosis and clubbing.The patient required a close follow-up because of complications that might develop on the long-term.
Keywords: Pachydermoperiostosis; Hypertrophic osteoarthropathy; pachyderma; hyperhidrosis; acropachia.
Introduction
PDP is the primary form of hypertrophic osteoarthropathy (HOA) which should be distinguished from the secondary form of HOA, which is much more frequent and mostly associated with severe pulmonary disease, bronchogenic carcinoma, lung empyema, bronchiectasis, congenital heart disease, and thyroid or GI malignancy [1]. It was first described by Friedreich [2] in 1868, who called it ‘Hyperostosis of the entire skeleton’. In 1907, Unna named the term ‘cutis verticis gyrate’ for thick, transversely folded skin of scalp and forehead [3]. In 1935, three dermatologists, Touraine, et al, [4] recognized this condition as a familial disorder with three forms: complete (periostosis and pachyderma), incomplete (without pachyderma) and the forme fruste (pachydermia with minimal skeletal changes). In 1965, Rimoin [5] observed affected persons in successive generations.
Jajic estimated the prevalence of the disease is 0.16%. [6,7] Symptoms usually appear around puberty, with a male to female ratio of 7:1, and males are severely affected [8]. In a review of 68 published families with PDP, including 204 patients, Castori et al [7] found that 37 families showed autosomal dominant inheritance and autosomal recessive inheritance was suggested in the remaining families. The main features are digital clubbing, skin changes (flushing, blanching, hyperhidrosis and hypertrophy) causing coarse facial features with thickening, furrowing and excessive oiliness of the skin of the face and forehead. Bone and joint involvement includes arthritis, arthralgia, periosteal new bone formation, subperiosteal ossification, acro-osteolysis and osteoporosis. Gastric hypertrophy, gastric ulcer and other endocrine abnormalities have been described. This condition progresses slowly for a few years and is self-limiting thereafter [9].
The pathogenesis of PDP is not fully known. The15-hydroxyprostaglandin dehydrogenase gene and thesolute carrier organic anion transporter family member2A1 have been found to be associated with PDP [10,11,12,13]. It is thought that increased levels of prostaglandin E2(PGE2) as a result of defective selective uptake acrossthe plasma membrane by solute carrier organic aniontransporter family member 2A1 and/or intracellular degradationby 15-hydroxyprostaglandin dehydrogenase iscentral to the pathogenesis of PDP [14,15,16]. Elevated PGE2 levels are hypothesized to induce cytokine-mediated tissue remodeling and vascular stimulation, leading to hyperhidrosis, acro-osteolysis, periostosis, arthritis, and pachyderma as seen in PDP patients [17].
Case Report
A 22 years old student from rural Bangladesh, presented to our outpatient department with the complaints of pain small joints of both hands and feet for 2 years. The pain was insidious in onset, throbbing in nature and not relieved by over-the-counter medications. There was neither morning stiffness nor back pain or sole pain. The patient also complained of profuse sweating, progressive enlargement of hands and feet and gradual coarsening of facial features. His family history was significant for consanguinity – his grandparents have a consanguineous relationship. There was otherwise no history of a similar illness in the family members, and this was the first time the patient sought medical attention for this issue. There was no history of scalp dandruff or rashes, and the patient denied having symptoms such as fatigue, eye redness, eye or mouth dryness, chest pain, or exertional dyspnea. There was no history of fever, palpitations, heat intolerance, tremors, trauma or fracture.
On examination, he had leonine facies with pronounced folds in the area of forehead, between the eyes, in the nasolabial grooves and on the chin, furrowing on his forehead skin (Figure 1). His nose was excessively enlarged with thickened skin folds. The development of the patient's skin folds was insidious and progressive. Clubbing of his fingers and toes (Figure 2 and 3) was noticed. Patient has profuse sweating and seborrhea in his axillae, hands, and feet. Cardiovascular, respiratory, neurological, and thyroid examination performed for the patient was otherwise unremarkable. There was no scalp dandruff, rashes, joint swelling, psoriatic nail changes, subcutaneous nodules, or eye redness noted on examination.
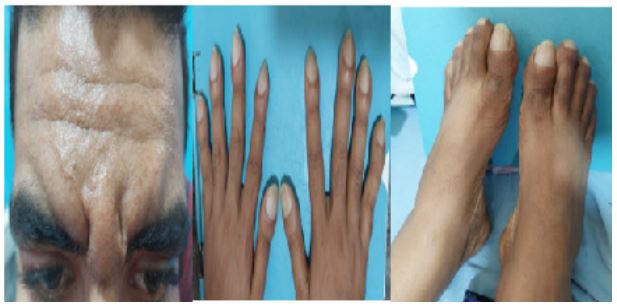
Figure 1,2 and 3: Furrowing of forehead, clubbing of fingers and toes with swollen both ankle joints
Laboratory analyses showed normal erythrocyte sedimentation rate, C-reactive protein, random blood sugar, serum calcium, liver function tests, renal function tests and thyroid function tests. Negative results were obtained forrheumatoid factor, anticyclic citrullinatedprotein (anti-CCP) antibodies,ANA and HLA-B27. As there was a suspicion ofacromegaly, performed an oral glucose tolerance test; the results of both of these tests were normal. Radiography of the skull showed mild cortical and sub periosteal thickening ( Figure 4).). X ray of tibia and fibula demonstrated irregular subperiosteal newbone formation and cortical thickening of tibia and fibula (Figure 5).The x-rays of bilateralhands showed soft tissue tumefaction, particularly in thedistal phalanges and periostitis and hyperostosis of metacarpaland proximal phalanges (Figure 6).Patient refused for skin biopsy and Urinary PGE2level was not available in our country. Based on pachydermia, digital clubbing, and typical radiologic findings of diffuse periostosis, a diagnosis of the complete form of pachydermoperiostosis was established.
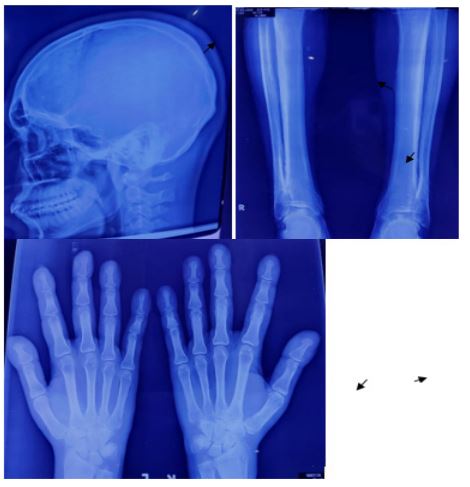
Figure 4,5 and 6: X-ray skull showed mild cortical and sub periosteal thickening (black arrow),X ray of tibia and fibula demonstrated irregular subperiosteal newbone formation and cortical thickening of tibia and fibula(black arrow),x-rays of bilateralhands showed soft tissue tumefaction, particularly in thedistal phalanges and periostitis and hyperostosis of metacarpal and proximal phalanges.
The patient stayed in our center for 7 days and wasmanaged with steroids (Prednisolone 7.5 mg PO, OD), oral bisphosphonate (risedronate 35 mg once weekly), oral propanthelene(15 mg twice daily)and isotretinoin ointment. The joint pain and swelling improved markedlywith treatment. He was subsequently dischargedwith outpatient follow-up scheduled 1 month later. On follow-up, itwas found that his joint pain and swelling was minimaland the pachyderma had reduced gradually since discharge.The patient experienced no relapses or complicationsfrom the condition or the medications. He refused for plastic surgical referral due to financial constraints.
Discussion
PDP or Touraine-Solente-Gole syndrome is the primary form of HOA. Although an autosomal dominant inheritance with incomplete penetrance and variable expression has been confirmed, both autosomal recessive and X-linked inheritance has been suggested [7]. PDP is related to mutations of the gene encoding for 15-hydroxyprostaglandin dehydrogenase (15HPGD) [10]. PDP patients have high levels of PGE2 and decreased levels of PGE-M (the metabolite of PGE2). PGE2 can mimic the activity of osteoblasts and osteoclasts, which may be responsible for the acro-osteolysis and periosteal bone formation [11]. PGE2 also has vasodilatory effects, which may be responsible for prolonged local vasodilation resulting in digital clubbing [11]. A familial history is found in 25 to 38 percent of patients. Our patient did not have family history.
PDP makes 3%‐5% of cases of hypertrophic osteoarthropathy and should be distinguished from the secondary formbefore a diagnosis of PDP is established [18]. The secondary form usually results from cardiopulmonary diseases (eg,bronchiectasis, cystic fibrosis, congenital heart diseases, andtuberculosis), hepatic diseases (eg, portal and biliary cirrhosis),gastrointestinaldiseases(eg,inflammatoryboweldisease andpolyposis), andcertain malignancies (eg, Hodgkin'sdisease,nasopharyngealcarcinoma, andchronicmyeloidleukemia).Clinically, insecondaryform,thecutaneousfindings(pachydermia,seborrhea, oiliness)are lessfrequentthanprimary PDP;theosteoarthropathy ismoresevere and painful,especiallywithcongenitalcyanoticheartdisease [19]. In secondary form due to neoplasia, only treatment of the underlyingillness causesimprovementofthe associatedsymptoms [20].
Other differential diagnoses include acromegaly, thyroid acropachy, van Buchem's disease (in which there is absence of clubbing and skin changes), psoriasis, and rheumatoid arthritis. Patientswithforme frusta have to be differentiatedfrom the rare hyperelasticity disorderssuch as Ehler‐Danlossyndrome, cutislaxa,Meretoga'ssyndrome,Marfan'ssyndrome, andpseudoxanthomaelasticum,whichmay causeforeheadfurrows [21].
In up to 30% of the patients, PDP presents as a hereditary disease with autosomal dominance of variable penetrance. Although pathogenesis iscurrentlyunknown, anincreased level of prostaglandin E2 whichmotivatestheoverexpression of the vascular endothelial growthfactor hasbeen proposed asamain factor. Duetohigh M:Fratio,X‐linkedtransmission androle oftestosteronehormonehavebeensuggested asotherfactors [22]. Recently, hydroxyprostaglandin dehydrogenase (HPGD) and solute carrier organicanion transporter family member 2A1 (SLCO2A1) weredescribed as pathogenic genes responsible for PDP. Whengermline SLCO2A1 mutations are detected, myelofibrosis,a life‐threatening complication, should be suspected and individualfollowed upperiodically. Alcoholicconsumption might be a contributing factor by alteration of prostaglandin metabolism [23]. Unfortunately, genetic testing for our reported case was not available at our center.
The diagnostic criteria for pachydermoperiostosis [9,24] are:
Major criteria: pachyderma, periostosis, finger clubbing.
Minor criteria: hyperhidrosis, arthralgia, gastric ulcer, cutis verticis gyrate, blepharoptosis, joint effusion, column-like legs, edema, seborrhea, acne, flushing. Our patient had all three major criteria i.e., hyperostosis, finger clubbing, and pachyderma. Martinez-Lavin [13] proposed the pathology for pachydermoperiostosis is due to increased amount of proliferation of collagen fibers from actively proliferating fibroblasts.
Pachydermia [25]—which affects the face and limbs—is the most frequent skin symptom.
The grading of pachyderma is as follows – grade 0:- absence, grade 1:- mild to moderate involvement (cutaneous thickening without puckering), grade 2:- severe (cutaneous thickening and puckering). Patients may also present with seborrhea (90% of cases), acne, folliculitis, dilated pores, hyperhidrosis of the palms and soles (44–67% of cases) may be associated with flushing, thickened eyelids (30–40% of cases), cutis vertices gyrate (24% of cases), and reduced facial and pubic hair.
Digital clubbing is seen in 89% of cases and nail bed capillary microscopy shows slight capillary enlargement and increased tortuosity. Arthritis is seen in 20–40% of cases26 and a joint effusion is seen in 41%. Irregular periosteal ossification affects predominantly the distal ends of long bones seen in 80–97% of patients.
A range of processes (some malignant) has been reported in association with pachydermoperiostosis. These include facial epidermoid carcinoma, [20] hypertrophic gastritis, peptic ulcer, gastric adenocarcinoma, [27] Crohn’s disease, and myelofibrosis [28]. As a consequence of increased soft tissue bulk and hyperostosis, complications may arise such as ptosis, compression of the nerve endings, hearing problems, kyphosis, arthrosis, osteonecrosis of the femoral head, and carpal tunnel syndrome. Variants of pachydermoperiostosis include Rosenfeld-Kloepfer syndrome (characterized by enlargement of the jaws, especially mandible, and of the hands and feet, nose, lips, tongue, and forehead, along with cutis vertices gyrata and corneal leukoma); Currarino idiopathic osteoarthropathy (an incomplete form of PDP seen in children and adolescents and characterized by the presence of eczema and sutural diastases); and a localized form with only the radiographic features of PDP in the lower extremities.
The diagnosis was confirmed by histopathological study.Histopathological examination of skin samples takenfrom patients with PDP shows epidermal acanthosis and hyperkeratosis,differentdegrees offibrosis andcapillaryectasia ofthedermis aswell as sebaceousglandhypertrophy.Bone biopsy showed cortical hyperostosis and thickening ofthe periosteum with bands of partially hyalinized connectivetissue in addition to vascular hyperplasia, with a reduction intrabecular bone. In cases with arthritis, the synovial membraneshowedvascularcongestionandstromal edema, lymphocyticandmonocyticinfiltration, andformationofsolitarylymphaticfollicles [29,30]. Radiographs of the hands and feetshow joint space narrowing, swelling in the soft tissues, andacro‐osteolysis of the distal phalanges. There is also symmetricalperiostosisthat ismoreprominent inthedistallowerlimbs [31].
This syndrome can be distinguished from acromegaly on the basis of clinical features and laboratory findings. In contrast to PDP, acromegaly presents clinically with larger bones in the face, skull and limbs, jaw prognathism, along with elevated insulin-like growth factor-1 levels and positive oral glucose tolerance test [32,33,34]. Acromegaly is often caused by a pituitary tumor, and thepotential manifestations of the tumor’s local compressionand hormonal disruption additionally help to distinguishit from PDP. In our patient, closer scrutiny in theclinical examination coupled with the negative biochemicalmarkers for acromegaly effectively allowed us to ruleout this differential.
The case presented here is a complete form of the syndrome, withthepresence ofmost ofthe clinical characteristic and radiologicalfindings. The patient hadsignificant joint involvement and severedigital clubbing, and the presence of bonyexcrescences was detected intheX‐rays ofhishands andfeet. Inthis case, cutisverticisgyrateaffectedonly hisforehead andsmallarea ofthescalp.Furthermore,althoughthissyndrome has astrong associationwithheredity, inthis case,therewas noreport ofrelativeswithsimilarcharacteristics.
No specific treatment exists; however, in most cases, PDP tends to stabilize over time. Conventional PDP drug treatments to decrease inflammation and pain include aspirin, NSAIDs and corticosteroids [35]. Rheumatologic symptoms can be improved by treatment with bisphosphonates, such as pamidronate or risedronate [35]. In isolated cases, tamoxifen was effective in PDP treatment, especially for bone and joint pain [35]. Retinoids are used to improve skin manifestations. Isotretinoin improves cosmetic features by inducing apoptosis within human sebaceous glands. As a result, the increase of connective tissue and hyperplasia of sebaceous glands is inhibited. Retinoids also decrease procollagen mRNA in fibroblasts, improving pachyderma and cutis verticis gyrate [36,37]. Colchicine can also improve articulr symptoms and skin manifestations such as folliculitis, and pachyderma [35]. The use of Botulinum toxin type A (BTX-A) may improve the leonine facies. Da costa et al reported the use of infliximab in a patient with refractory arthritis [38]. Surgical methods, including facelifts and facial rhytidectomy, have also been used to improve facial appearance [39].
Conclusion
The diagnosis of PDP is based on the combination of digital clubbing, periostitis and pachyderma with the absence of any cardiovascular, pulmonary, liver, intestinal and mediastinal diseases.Since PDP is a disease associated with stigmatization and a consequent reduction in the patient's quality of life, diagnosis of its various clinical forms and regular follow‐up by a team that includes a plastic surgeon, rheumatologist, and orthopedic are factors of ultimateimportance.Clinical presentations of PDP can be confused with secondaryhypertrophic osteoarthropathy, psoriatic arthritisrheumatoid arthritis, thyroid acropachy, and acromegaly Awareness of the significance of clubbing under these circumstances is likely to prevent misdiagnosis.
Conflict of interest: None declared.
References
- Vogel A, Goldfischer S. pachydermoperiostosis: Primary or idiopathic hypertrophic osteoarthropathy. Am J Med. 1962; 33: 166-187.
- Friedrich N. Hyperostose des gesammten skelettes: Virchows. Arch Pathol Anat. 1868; 43: 83.
- Unna PG. Cutis verticis gyrate. Monastsh PRAKTIS Dermatol. 1907; 45: 227–233.
- Touraine A., Solente G., Gole L. Un syndrome osteodermopathique: la pachydermie plicaturee avec pachyperiostose des extremites. Presse Med. 1935; 43: 1820–1824.
- Rimoin DL. Pachydermoperiostosis (idiopathic clubbing and periostosis). Genetic and physiologic considerations. New Eng J Med. 1965; 272: 923–931.
- Jajic I, Jajic Z. Prevalence of primary hypertrophic osteoarthropathy in selected population. Clin Ex Rheum. 1992; 10: 73.
- Castori M, Sinibaldi L, Mingarelli R, Lachman RS, Rimoin DL, Dallapiccola B. Pachydermoperiostosis: an update. Clin Genet. 2005; 68: 477–486.
- Reginato AJ, Shipachasse V, Guerrero R. Familial idiopathic hypertrophic osteoarthropathy and cranial suture defects in children. Skel Radiol. 1982; 8: 105–109.
- Martinez-Lavin M. Digital clubbing and hypertrophic osteoarthropathy: a unifying hypothesis. J Rheumatol. 1987; 14: 6–8.
- Uppal S, Diggle CP, Carr IM, Fishwick CW, Ahmed M, Ibrahim GH, Helliwell PS, Latos-Bieleńska A, Phillips SE, Markham AF, Bennett CP. Mutation hydroxyprostaglandin dehydrogenase cause primary hypertrophic osteoarthropathy. Nat Genet. 2008; 40(6): 789.
- Yüksel-Konuk B, Sırmacı A, Ayten GE, Özdemir M, Aslan İ, Yılmaz-Turay Erdoğan Y, Tekin M. Homozygous mutations in the 15hydroxyprostaglandin dehydrogenase gene in patients with primary hypertrophic osteoarthropathy. Rheumatol Int. 2009; 30(1): 39–43.
- Zhang Z, Xia W, He J, Zhang Z, Ke Y, Yue H,et al. Exome sequencing identifies SLCO2A1 mutations acause of primary hypertrophic osteoarthropathy. Am J Hum Genet. 2012; 90(1): 125–32.
- Sasaki T, Niizeki H, Shimizu A, Shiohama A, Hirakiyama A, Okuyama T, Kabashima K, Otsuka A, Ishiko A, Tanese K. Identification of mutations prostaglandin transporter gene SLCO2A1 and its phenotype–genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol 2012; 68(1): 36–44.
- Tai HH, Cho H, Tong M, Ding Y. NAD+-linked 15-hydroxyprostaglandin dehydrogenase: structure and biological functions. Curr Pharm Des. 12(8): 955–62.
- Guo T, Yang K, Liu L, Tan ZP, Luo H. Identification of two novel mutations the SLCO2A1 prostaglandin transporter gene in a Chinese patient with primary hypertrophic osteoarthropathy. Mol Med Rep. 2017; 15(5): 297713.
- Coggins KG, Coffman TM, Koller BH. The Hippocratic finger points the blame at PGE2. Nat Genet. 2008; 40: 691–2.
- Bergmann C, Wobser M, Morbach H, Falkenbach A, Wittenhagen D, L, Ott H, Zerres K, Girschick HJ, Hamm H. Primary hypertrophic osteoarthropathy with digital clubbing and palmoplantar hyperhidrosis caused by 15-PGHD/HPGD loss-of-function mutations. Exp Dermatol. 20(6): 531–3.
- El Aoud S, Frikha F, Snoussi M. Bilateral ptosis as a presentingfeature ofprimaryhypertrophicosteoarthropathy(pachydermoperiostosis): a case report.Reumatismo.2014; 66(3): 249–253.
- Rastogi R, Suma GN. Pachydermoperiostosis or primary hypertrophicosteoarthropathy: arareclinicoradiologic case.Indian JRadiolImaging.2009; 19(2): 123–126.
- Lee S‐C, Moon H, Cho D, et al. Pachydermoperiostosis with cutaneoussquamous cellcarcinomas.IntDermatol.1998; 37: 687–700.
- Rajan TM, Sreekumar NC, Sarita S, Thushara KR. Touraine solente golesyndrome:the elephant skin disease.Indian JPlastSurg.2013; 46(3): 577–580.
- Rahaman SH, Kandasamy D, Jyotsna VP. Pachydermoperiostosis: incomplete form, mimicking acromegaly. Indian J Endocrinol Metab. 2016; 20: 730–731.
- Alnimer Y, Subedi S, Dawood T, Bachuwa G. Primary idiopathic osteoarthropathy: could it be related to alcoholism? Case Rep Rheumatol. 2017; 2017: 2583762.
- Matucci-Cerinic M, Lotti T, Jajic I, Pignone A, Bussani C, Cagnoni M. The clinical spectrum of pachydermoperiostosis. Medicine. 1991; 70: 208–214.
- Kabi F, Mkinsi O, Janani S, Raissouni N. Pachydermoperiostosis. A case report. J Intern Med. 2006; 27: 710–712.
- Schumacher HR. Hypertrophic osteoarthropathy: rheumatologic manifestations. Clin Exp Rheumatol. 1992; 10: 35–40.
- Ikeda F, Okada H, Mizuno M. Pachydermoperiostosis associated with juvenile polyps of the stomach and gastric adenocarcinoma. J Gastroenterol. 2004; 39: 370–374.
- 28.Bachmeyer C, Blum L, Cadranel JF, Delfraissy JF. Myelofibrosis in a patient with pachydermoperiostosis. Clin Exp Dermatol. 2005; 30: 646–648.
- Mattuci‐Cerinic M, Lotti T, Jajic I, Pignole A, Bussani C, Cagnoni M. The clinical spectrum of pachydermoperiostosis (primary hypertrophic osteoarthropathy). Medicine (Balt). 1991; 70: 208–214.
- Auger M, Stavrianeas N. Pachydermoperiostosis. OrphanetEncyclopedia.2004; 1–8.
- Guerini MB, Barbato MT, deSá NB, Nunes DH, Zeni PR.Pachydermoperiostosis – the complete form of the syndrome. AnBras Dermatol. 2011; 86(3): 582–584.
- Glick J, Kaur RR, Taylor G. Pachydermoperiostosis vs. acromegaly in a patientwith cutis verticis gyrata. J Clin Exp Dermatol Res. 2012; 3(142): 2.
- Lugo G, Pena L, Cordido F. Clinical manifestations and diagnosis ofacromegaly. Int J Endocrinol. 2012; 2012: 540398.
- Abdullah NRA, Jason WLC, Nasruddin AB. Pachydermoperiostosis: a rare mimicker of acromegaly. Endocrinol Diabetes Metab Case Rep. 2017; 2017: 17–0029.
- Gomez RN, Ibanez RJ, Gonzalez PM. Primary hypertrophic osteoarthropathy. Report of 2 familial cases with literature review. Rheumatol Clin. 2009; 5: 259-63.
- Athappan G, Unnikrishnan A, Chengat V, et al. Touraine Solente Gole syndrome: the disease and associated tongue fissuring. Rheumatol Int. 2009; 29: 1091-93.
- Park YK, Kim HJ, Chung KY. Pachydermoperiostosis: trial with isotretinoin. Yonsei Medical Journal 1988; 29: 204-7.
- DaCosta FV, de Magalhães Souza Fialho SC, Zimmermann AF, Neves FS, Werner de Castro GR, Pereira IA. Infliximab treatment in pachydermoperiostosis: a rare disease without an effective therapeutic option.J Clin Rheumatol. 2010; 16: 183–184.
- Góez Rodríguez N, Ibáñez Ruán J, González Pérez M. Primary hypertrophic osteoarthropathy (pachydermoperiostosis). Report of 2 familial cases and literature review. Reumatol Clin. 2009; 5(6): 259–263.

