Case Report - Volume 2 - Issue 5
Adenocarcinoma of the Gallbladder: A case of a rare metastasis to the thyroid
Stullich R1; Schammel C2; Schammel DP2; Devane AM3; Trocha SD4*
1University of South Carolina School of Medicine Greenville, Greenville SC, USA.
2Pathology Associates, Greenville SC, USA.
3Department of Radiology, Prisma Health Upstate, Greenvile SC, USA.
4Department of Surgery, Prisma Health Upstate, Greenville SC, USA.
Received Date : Sep 05, 2022
Accepted Date : Oct 13, 2022
Published Date: Oct 31, 2022
Copyright:© Steven D Trocha 2022
*Corresponding Author : Steven D Trocha, GI Oncology Division Chief Department of Surgery, Prisma Health Upstate, Greenville SC 29605,USA.Tel: 864-455-1200
Email: Steven.trocha@prismahealth.org
DOI: Doi.org/10.55920/2771-019X/1279
Abstract
Introduction: Gallbladder cancer is a rare and aggressive form of gastrointestinal malignancy, typically remaining asymptomatic making early diagnosis uncommon. Five year overall survival in patients with distant metastasis is 2% with a median survival of 5.8 months, with metastases typically identified in the liver and lung. Here we present the fourth case of metastases to the thyroid gland.
Case report: A 63-year-old male presented with a left-sided neck mass. CT revealed a necrotic node as well as a large calcified nodule of the right thyroid. PET-CT identified a hypermetabolicbolic left neck mass, a right thyroid lobe nodule, and a hypermetabolic lesion in the periphery of the liver. Biopsies of the left neck and right thyroid lesions were consistent with neuroendocrine metastatic carcinoma non-specific as to site of origin. A total thyroidectomy and left cervical lymphadenectomy identified poorly differentiated neuroendocrine carcinoma. Surgical resection of the gallbladder revealed the primary lesion: poorly differentiated gallbladder adenocarcinoma with neuroendocrine features (pT2 pNX pLV1 pM1).
Conclusion: Gallbladder cancer metastasis to the thyroid is rare. Hoarseness and dysphasia may indicate not only a primary thyroid lesion but also a rare metastasis from another primary site. Thorough imaging is essential to determine metastatic spread and possibly identify an unknown primary. If histologic evaluation of the thyroid lesion reveals adenocarcinoma, a thorough abdominal evaluation is warranted. Although the outcome of metastatic disease to the thyroid from the gallbladder is poor, the identification of the primary site is important in the targeted multi-modality management of this rare presentation.
Keywords: Gallbladder cancer; adenocarcinoma with neuroendocrine features; thyroid metastasis; comprehensive literature review.
Introduction
Gallbladder cancer is a rare and aggressive form of gastrointestinal malignancy, accounting for 1.2% of new cancer diagnoses worldwide in 2018 and 1.7% of cancer deaths [1]. It is estimated that 12,130 new cases of cancer involving the gallbladder and large bile ducts will be diagnosed in the United States in 2022, with 4 in 10 of these cases being gallbladder cancer [2]. The most common histologic subtype of gallbladder carcinoma is adenocarcinoma, comprising 90% of all cases. [3,4] With an estimated two thirds of cases and deaths occurring in older women (70 years of age), [1,3] gallbladder cancer has been associated with a history of gallstones, [5,6] an increased BMI, [7] tobacco use, chronic inflammation, bacterial infection, and polyps [8].
As the disease typically progresses in an asymptomatic manner, early diagnosis of gallbladder cancer is uncommon, with only 20% being diagnosed when the disease is still confined to the gallbladder. [2,3] Five-year overall survival in patients with distant metastasis is 2% with a median survival of 5.8 months;[2,4] metastases may occur to a wide variety of locations, most often the peritoneum, liver, and lung [9] but also, but rarely, have been observed in unusual locations such as the thyroid gland. [10,11,12] Here we report a case of unusual metastasis of gallbladder cancer to the thyroid with a comprehensive review of the other three cases reported in the literature.
Case Report
A 63-year-old male presented to his primary physician at an annual wellness visit with left-sided neck mass that had been noted to be present for about two weeks, was tender to palpation, and increasing in size. As there was concern for an abscess, an ultrasound was completed to appropriately characterize the lesion. Imaging revealed a solid mass with lobular margins and peripheral blood flow. As these findings were concerning for a non-infectious etiology, a CT of the neck with contrast was performed which demonstrated a partially necrotic level II B node on the left as well as a large calcified nodule of the right thyroid, suspicious for possible malignancy. No other definitive mucosal lesions were noted. The patient was referred to oncology for an evaluation of a potential thyroid cancer. Labs values revealed an abnormal CEA 6.4 (0.0-3.0 ng/mL) and thyroglobulin 65.2 (2.8-40.9 ng/mL); TSH, total T4, thyroglobulin antibody, uric acid and LDH were all normal. PET-CT revealed a hypermetabolic left neck mass and right thyroid lobe nodule suggestive of malignancy. The scan also noted a hypermetabolic lesion in the periphery of the liver concerning for malignancy (Figure 1); however, it could not be definitively determined if the lesion was located in the liver along the cephalad margin of the gallbladder fundus or if the lesion was a mass of the gallbladder fundus extending to the liver surface. An FNA of the left neck and right thyroid lesions were consistent with metastatic carcinoma of likely neuroendocrine origin. Pathology from a CT-guided biopsy of the liver identified poorly differentiated carcinoma with neuroendocrine features that were non-specific as to site of origin (Figure 2a). Synaptophysin immunohistochemical staining was performed to confirm neuroendocrine origin (Figure 2b).
One month after the initial primary care visit, the patient began complaining of hoarseness and difficulty swallowing; surgery was consulted. A total thyroidectomy and selective left cervical lymphadenectomy were completed. The thyroid illustrated poorly differentiated neuroendocrine carcinoma (Figure 3); no demonstratable nodal tissue was noted in the left lateral neck mass but was identified as poorly differentiated neuroendocrine carcinoma metastatic to connective tissues (Figure 4). All nine sampled lymph nodes were negative for metastatic disease. Histologic staining revealed a negative calcitonin and CEA expression which favored a diagnosis of metastatic disease of unknown origin.
Presuming an additional metastasis, the multidisciplinary conference suggested surgical resection of the liver lesion. A pre-operative CT liver was notable for interval enlargement. A robotic segment 4B/5 segmentectomy and cholecystectomy was performed. Histology identified the primary of a stage IV poorly differentiated gallbladder adenocarcinoma with neuroendocrine features (Figure 5a and 5b). The apparent liver lesion was identified as an extension of the gallbladder tumor and not a metastasis. As metastatic involvement included the thyroid gland, and connective tissue of the neck the final pathologic stage was pT2 pNX pLV1 pM1.
The patient was unable to be enrolled in a clinical trial due to the neuroendocrine features of his malignancy and was started on adjuvant chemotherapy which included gemzar, cisplatin, and abrazane. After three cycles of chemotherapy, follow-up scans revealed no evidence of disease warranting monthly surveillance off of chemotherapy. Within a month, cystic nodules arising from the subcutaneous fat of the trunk were noted; imaging revealed additional lesions on both adrenal glands and retroperitoneal lymph nodes. A wide local excision (WLE) of a truncal lesion revealed metastatic soft tissue morphologically consistent with the patient’s known gallbladder primary. A rare tumor trial was suggested; however, insurance failed to approve so monthly surveillance was continued. Progression of all sites of metastases were noted three months following the WLE. The patient switched insurance carriers and was enrolled in a rare tumor protocol (tremelimumab and durvalumab) with monthly surveillance showing stable disease. At week 16 of the protocol, the patient presented with weakness and confusion; treatment was terminated and the patient was referred to hospice. The patient expired 19 months post-diagnosis.
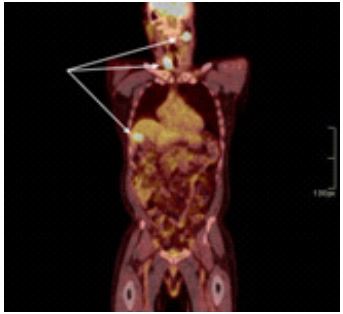
Figure 1: PET scan. The left level IIB cervical lymph node, right thyroid and gallbladder/liver uptake are noted.
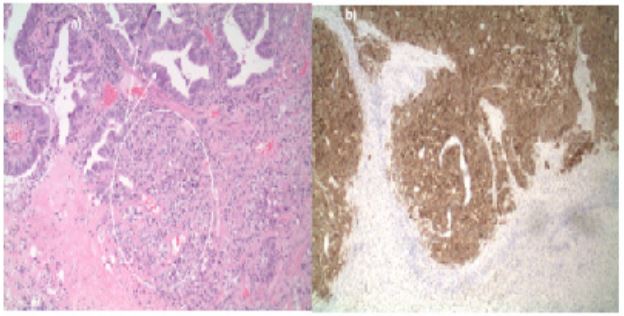
Figure 2: Liver Biopsy. a) Histology. Poorly differentiated carcinoma with neuroendocrine differentiation example is circled. Necrosis is also noted. b) Synaptophysin immunohistochemical staining highlights neuroendocrine differentiation.
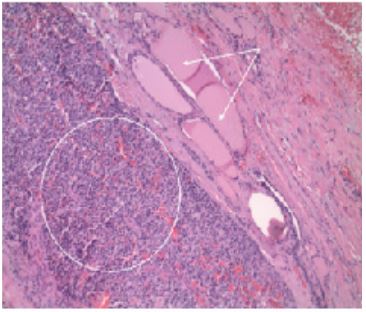
Figure 3: Thyroid metastasis. Poorly differentiated carcinoma with endocrine features (circled) the thyroid follicular epithelium with colloid (arrows).
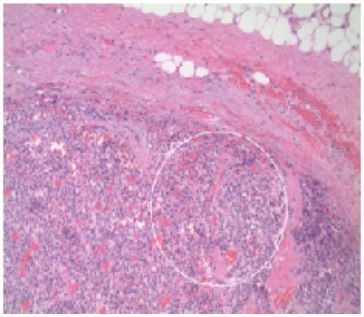
Figure 4: Cervical lymph node metastasis. Poorly differentiated carcinoma with endocrine features beneath the fibrous connective tissue and fat. No demonstrable nodal tissue.
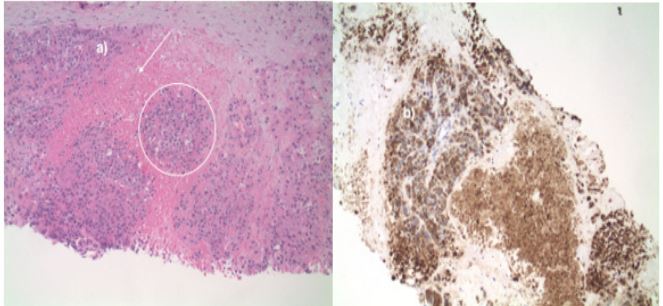
Figure 5: Gallbladder resection. a) Histology. High grade dysplasia within overlying glandular epithelium (arrows) with underlying associated poorly differentiated carcinoma with neuroendocrine differentiation (example circled). b) Synaptophysin immunohistochemical stain. Neuroendocrine differentiation is highlighted.
Discussion
Gallbladder cancer is a rare but highly malignant cancer, which is often diagnosed at a metastatic stage due to its asymptomatic manifestation. [2] Adenocarcinoma is the most common histologic variant of gallbladder cancer, comprising 90% of cases; further classification into subtypes notes adenocarcinoma NOS as the most common subtype (68% of all cases) with adenocarcinoma with neuroendocrine features comprising only 2% of cases. [3] Here we present a unique case of metastatic gallbladder adenocarcinoma with neuroendocrine features with initial presentation as a left neck mass identified as metastasis to the thyroid gland.
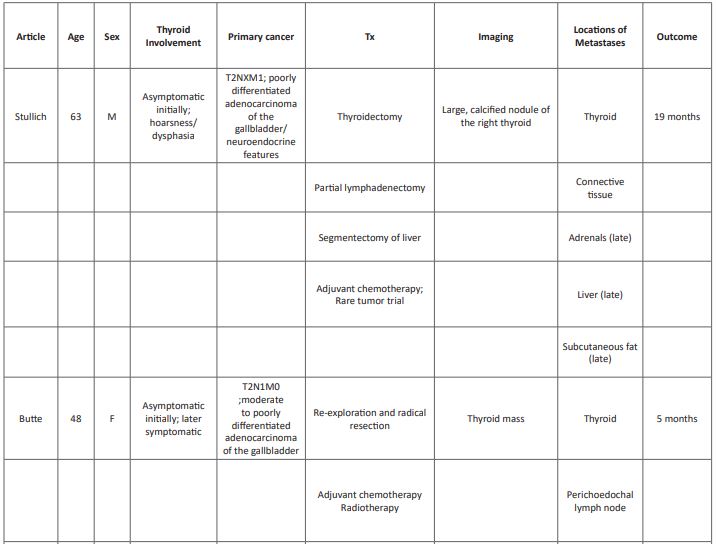
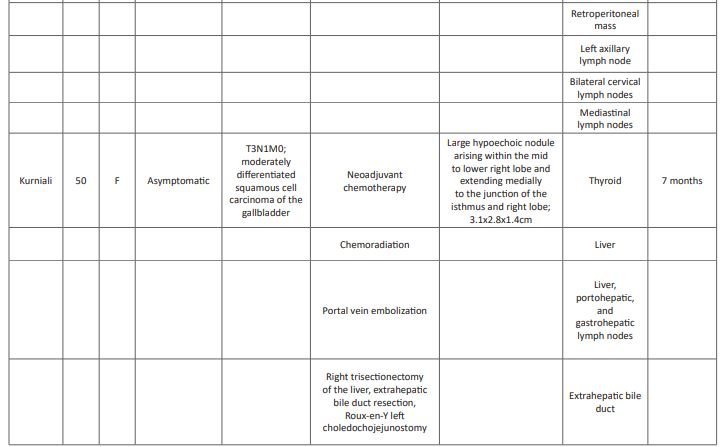
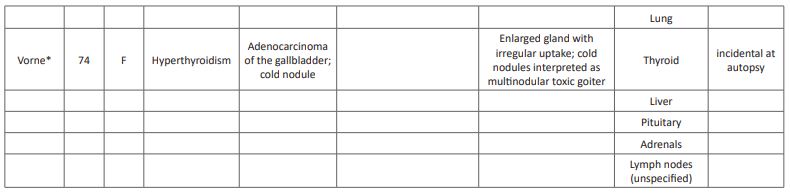
A comprehensive literature review revealed only three other reports of gallbladder carcinoma with thyroid metastases (Table 1). [10,11,12] Mean age of this cohort is 59 (range 48-74), younger than those with adenocarcinoma of the gallbladder (mean age 72) [2]. All previously reported cases occurred in females, with our patient is the only reported male. Interestingly, it has been suggested that estrogen may play a role in gallbladder cancer and metastases of cancers in general to the thyroid as the prevalence of both entities is higher in women; [13,14,15] however, these data are controversial. The unique pattern of metastasis to the thyroid in this small subset may suggest that certain histologic subtypes have a predilection for the thyroid gland. However, the cases presented here represent a varied histology and grade: moderate to poorly differentiated adenocarcinoma (2), moderately differentiated squamous cell carcinoma (1) and poorly differentiated adenocarcinoma with neuroendocrine features (1; Table 1). Likewise, there is an absence of a pattern of other metastatic sites between these patients, with previously reported patients have had greater number of metastatic sites at the time of diagnosis of the thyroid metastasis: distant spread to the lung, axillary nodes, cervical nodes, and mediastinal nodes (Table 1). Our patient had only one other site of metastatic involvement upon diagnosis, pathologically identified as connective tissue of the neck. While this may represent a replaced lymph node, all other sampled lymph nodes were negative, and no other nodes were noted on PET-CT; the apparent liver lesion was identified as an extension of the gallbladder and not a metastasis. With disease progression, metastatic disease in our patient became widespread, involving the adrenals, liver and truncal and limb subcutaneous fat. The variability within these cases regarding histology and metastatic spread causes difficulty in effectively predicting the biology and behavior of these lesions.
Due to the rarity of gallbladder cancer presenting with a metastasis to the thyroid, and the extent of disease at the time of diagnosis, treatments have included surgical resection of both the gallbladder and the thyroid, adjuvant chemotherapy and radiation therapy (Table 1); however, survival was reportedly short, only 4 and 7 months (Butte and Kurniali, respectively). Due to the lack of obvious lymph node involvement in our patient, surgical resection and adjuvant chemotherapy were offered and, within a few months, the patient was classified with no evidence of disease. However, within a month of the completion of traditional chemotherapy and initiation of surveillance only, other metastatic lesions manifested, suggesting an aggressive biology. While a rare tumor protocol utilizing immunotherapy was available, the lack of insurance coverage precluded initiation for five months, during which time the disease progressed. Once the rare tumor protocol was initiated, the disease stabilized; however, the underlying disease had progressed and the patient expired at 19 months post-diagnosis.
While resection is still the curative option for gallbladder cancer, this strategy is limited as most patients present with metastatic disease [16,17]. Due to the rarity of this disease, especially in the cases reviewed here, and the nature of this unusual presentation, traditional chemotherapy has not been shown to be effective. However, more recently, as the biology of gallbladder metastases has been more thoroughly investigated [19] and genetic analysis of tumors has promoted the development of additional therapeutic strategies such as targeted immunotherapy, as was noted in our patient, there is promise of controlling the progression of disease [18]. Thus, there is an essential role for bioanalysis for all rare tumors and early employment of targeted therapies to optimize patient care and influence outcomes.
Table 1: Comprehensive literature review of gallbladder cancer metastases to the thyroid.
Conclusion
Gallbladder cancer typically presents asymptomatically; the thyroid is an unusual site for metastatic spread for most cancers and is an extremely rare site for metastatic gallbladder metastasis. Thorough imaging to identify extent of disease is an essential first step in the establishment of a differential; histologic evaluation and bioanalysis of lesions is essential for aids in a definitive diagnosis of subtype as well as identification of potential targetable mutations in this rare metastatic presentation. Consideration and implementation of current targeted therapies or rare tumor protocols for these uncommon presentations is essential to optimize patient treatment and outcomes.
Declarations:
Ethical approval and consent to participate:
Informed consent was obtained from all individual participants included in the
Study. This case study was considered exempt dated 4/8/2020 due to the universal consent used at our institution. IRB protocol number is PRO00099213 of Health Sciences South Carolina.
Funding: No funds, grants, or other support was received for this work.
Conflicts of interest: All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68: 394–424.
- https://www.cancer.net/cancer-types/gallbladder-cancer/statistics; accessed 9/1/2022
- Henley SJ, Weir HK, Jim MA, Watson M, & Richardson LC. Gallbladder Cancer Incidence and Mortality, United States 1999-2011. Cancer Epidemiology Biomarkers & Prevention. 2015; 24: 1319–1326.
- Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). Journal of Clinical Oncology. 2007; 25: 4648–4648.
- Zatonski WA, Lowenfels AB, Boyle P, et al. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997; 89: 1132–1138.
- Lowenfels AB, Walker AM, Althaus DP, Townsend G, Domellöf L. Gallstone growth, size, and risk of gallbladder cancer: an interracial study. Int J Epidemiol. 1989; 18: 50–54.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. The New England Journal of Medicine. 2003; 348:1625–1638.
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014; 6: 99‐109.
- Miller G, Jarnagin WR. Gallbladder carcinoma. Eur J Surg Oncol. 2008; 34: 306–12.
- Butte JM, Marin L, Meneses M, et al. Thyroid Metastases from Gallbladder Cancer. J Gastrointest Surg. 2009; 13:1389–91.
- Kurniali PC, Kavuturu S, Caliman N, Al-Janadi, A. Thyroid Metastases from Squamous Cell Carcinoma of Gallbladder. J Gastrointest Canc. 2014; 45: S82-S84.
- Vorne M and Jarvi K. Metastatic tumors detected as cold nodules on the thyroid scan. The British Journal of Radiology. 1987; 61: 329-330.
- Gupta P, Agarwal A, Gupta V, et al. Expression and Clinicopathological Significance of Estrogen and Progesterone Receptors in Gallbladder Cancer. Gastrointest Cancer Res. 2012; 5(2):41-47.
- Chun AY, Tran TB, Brumund KT, Weisman RA, Bouvet M. Metastases to the thyroid: a review of the literature from the last decade. Thyroid. 2012; 22(3): 258-268.
- Hryciuk B, Pęksa R, Bieńkowski M. et al. Expression of Female Sex Hormone Receptors, Connective Tissue Growth Factor and HER2 in Gallbladder Cancer. Sci Rep 2020; 10: 1871. [DOI: 10.1038/s41598-020-58777-y].
- Baiu I, Visser B. Gallbladder Cancer. JAMA. 2018; 320: 1294. [DOI: 10.1001/jama.2018.11815].
- Hickman L, Contreras C. Gallbladder Cancer: Diagnosis, Surgical Management, and Adjuvant Therapies. Surg Clin North Am. 2019; 99: 337-355. [DOI: 10.1016/j.suc.2018.12.008].
- Song X, Hu Y, Li Y. et al. Overview of current targeted therapy in gallbladder cancer. Sig Transduct Target Ther 2020; 5: 230. [DOI: 10.1038/s41392-020-00324-2].
- Yang Y, Tu Z, Ye C. et al. Site-specific metastases of gallbladder adenocarcinoma and their prognostic value for survival: a SEER-based study. BMC Surg 2021; 21: 59. [DOI: 10.1186/s12893-021-01068-8].

