Research Article - Volume 2 - Issue 6
Evaluation of Nodules in 40 Thyroidectomy Cases with CD56 Immunohistochemistry
Tülay Diken Allahverdi1* ; Gülden Yıldız2; Hatice Beşeren2; Yasemen Adalı İzmir3; Yalçın Polat4
1University, Faculty of Medicine, Department of General Surgery, Kars, Turkey.
2University, Faculty of Medicine, Department of Pathology, Kars, Turkey.
3University of Economics Faculty of Medicine, Department of Pathology İzmir, Turkey.
4Avicenna Laboratory, Department of Pathology, Ankara, Turkey.
Received Date : Sep 30, 2022
Accepted Date : Oct 24, 2022
Published Date: Nov 09, 2022
Copyright:© Tülay Diken Allahverdi 2022
*Corresponding Author : Tülay Diken Allahverdi, University, Faculty of Medicine, Department of General Surgery, Kars, Turkey.
Email: drtulaydiken@hotmail.com
DOI: Doi.org/10.55920/2771-019X/1288
Abstract
Background/Objective: Thyroid nodules are palpable, clinically undetectable lesions that can be found on ultrasound imaging. It is difficult to differentiate benign and malignant with ultrasound imaging. Fine needle aspiration is applied to these lesions for clinical, radiological and pathological examination to differentiate benign and malignant lesions. Immunohistochemical staining is performed for differential diagnosis during pathological examination. One of them is CD56 immunohistochemical marker. In this study, we aimed to evaluate the diagnostic role of CD56 immunohistochemistry in the differential diagnosis of lesions seen in various thyroid diseases.
Method: Forty cases with thyroid nodules who underwent thyroidectomy between 2020-2021 in the Department of Medical Pathology, Faculty of Medicine, xxx University were scanned from the pathology system and included in the study. Hematoxylin-eosin stained slides of the cases, which were made during routine service, were examined under a light microscope and paraffin blocks suitable for research were selected. A section of 3-4 micron thick was cut from the determined paraffin blocks and CD56 immunohistochemistry was applied to the slides. Statistical analyzes were made in SPSS 16.0 package program.
Results: According to the results of CD56 immunohistochemistry staining; 87.8% of papillary thyroid carcinoma, 79.1% of follicular thyroid carcinoma, 11.9% of follicular adenoma, 25.5% of benign follicular lesions and 19.6% of hashimoto thyroid. CD56 expression loss was found to be 1.6% in thyroid tissues without lesions.
Conclusion: Using CD56 is both a sensitive and specific method to differentiate follicular lesions seen in the thyroid tissue from papillary thyroid carcinomas. However, using CD56 marker together with other combinations may be more useful in differential diagnosis.
Keywords: CD56; papillary thyroid carcinoma; thyroid neoplasms; thyroid nodüle.
Introduction
Thyroid nodules are generally seen in 5% of patients and are detected incidentally [1]. Non-palpable, unrecognized thyroid nodules can be detected at a rate of 20-67% along ultrasound scanning [1,2]. Approximately 5-24% of detected nodules are diagnosed as malignancy [3]. Papillary cancer is seen in 80% of thyroid malignancies [4].
Chan and Saw [5] detected the microscopic features of thyroid lesions with routine hematoxylin and eosin (H & E) stained sections [4]. They stated that the evaluation of nuclear properties of PTC is highly depend on tissue processing like thickness of sections, fixation time and fixative type [5]. Nuclear features may be similar in benign and malignant papillary and follicular lesions [6]. Reactive atypia secondary to inflammation develops in Hasimoto's thyroiditis and lymphocytic thyroiditis [7]. This is similar to the morphological findings in papillary carcinoma [8]. If nuclear features are not detected adequately in the follicular variant and other variants of PTC [9,10], serious problems may arise in the differential diagnosis of thyroid cancers and benign lesions of the thyroid [11]. Diagnosis of noninvasive papillary thyroid carcinoma (FVPTC), which is the encapsulated follicular variant versus follicular adenoma, may cause difficulties in differential diagnosis among pathologists [5,12].
In order to avoid the confusions mentioned above, additional tests may help to reach the correct diagnosis [13]. Some immunohistochemical markers are important in terms of differential diagnosis(14). CD56 is among the most frequently used markers [15]. Although CD56 shows diffuse membranous staining, it is found in follicular epithelial cells of the normal thyroid [16,17]. It has been reported that the expression of CD56 is low or absent in PTC in immunohistochemical methods and/or analysis using PCR [18]. In this study, our aim is to examine the differences in CD56 expression, which is a marker that distinguishes the pathological features of the cases with variants of PTC from follicular thyroid lesions.
Materıal/ Method
The aim of this retrospective study was to determine the specificity and sensitivity of CD56 expression in thyroid lesions. In the study, 40 cases with thyroid nodules who underwent thyroidectomy inthe Department of General Surgery between the years 2020-2021 included in the study. This study was carried out with the approval of the local ethics committee of the xx University Faculty of Medicine number 80576354-050-99/202 dated july 01/2021. Consent form was obtained from the patients participating in our study. The cases who had no archive material in the Department of Pathology and/ or missing information excluded from the study. Also, cases other than bilateral thyroidectomy, cases with metastatic lesions to distant sites and recurrent lesions were excluded.
Hematoxylin-eosin stained slides of the cases, which were made during routine evaluation, were examined under a light microscope and paraffin blocks suitable for research were selected. CD56 immunohistochemistry was applied by taking a section from the determined paraffin blocks to 3-4 micron thick adhesive slides. Statistical analyzes were made in SPSS 16.0 package program.
Immunohistochemical Analysis
Tissue sections taken on slides were kept in an oven at 56 degrees overnight. The next day, the sections were kept in three separate xylenes for 5 minutes. Then, they were kept in graded alcohols for 5 minutes and washed in distilled water for 1 minute. It was boiled in 10% citrate buffer (Sigma-Aldrich, Ph 6.0) solution for 10 minutes. The lid of the vessel containing the boiled sections was opened and kept at room temperature for 20 minutes. After 20 minutes, the sections were rinsed with distilled water and kept in 10% hydrogen peroxide solution for 10 minutes, then washed again in distilled water and kept in W block (Thermo) for 5 minutes. At the end of the period, the primary CD56(Thermo NCAM-1/Ab-2)antibody was dripped onto the sections by shaking the W block on them without washing. The antibody was incubated for 60 minutes. After incubation, washing was done in distilled water for 10 minutes. Then, it was passed to the secondary antibody stage and kept in biotin (Thermo) solution for 20 minutes, washed in distilled water for 5 minutes and kept in streptavidin (Thermo) solution for 20 minutes. After washing in distilled water for 5 minutes, it was incubated in DAP chromogen (Thermo) for 7 minutes and washed. Finally, after 5 minutes of staining in Mayer's hematoxylin (Bio-Optica), it was passed through alcohol and xylene and closed with a mount.
Evaluation of Immunohistochemical Staining
Evaluations of the paints made, 10x, 20x and 40x selections were made. Membranous staining was accepted as positive (+). 10% or more staining was accepted as positive (+). In PTC cases, cytoplasmic staining was accepted as 'negative (-)'. The stained sections were examined by two observers and the smallness of the cases was judged by one observer on one occasion.
Statıstıcal Analysıs
Statistical Package for Social Sciences (SPSS) software was used for statistical analysis. The chi-square test was used to examine the relationship between the data.
Results
The ratio of males and females in our patients was 2.9. The mean age was 42.4 ± 17.0 (8-85). In our study, the age statistical result between PTC and non-PTC groups was P=0.475. The statistical result in terms of gender was found to be P=0.087. It was not found statistically significant in terms of age and gender. Of the 40 patients included in this study, 13 follicular lesions (9 follicular adenomas, 3 Hurthle cell adenomas, 1 follicular carcinoma), 12 multinodular goiter, 1 Hurthle cell carcinoma, 2 Hashimoto's thyroiditis, and 12 PTC were detected. Capsular invasion was observed in 9 of the 40 patients examined, and lymph node involvement was detected in 2 patients.
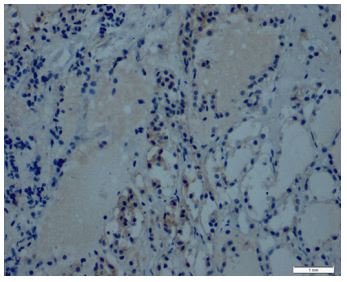
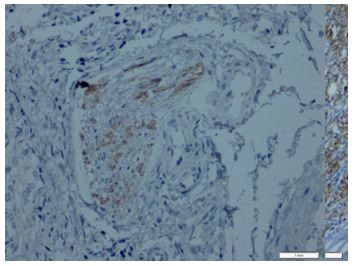
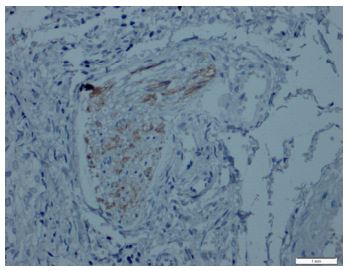
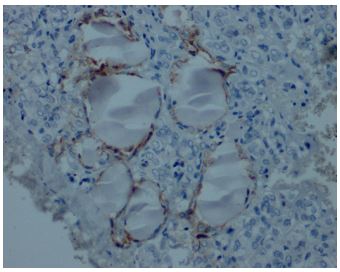
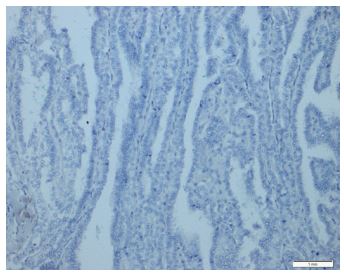
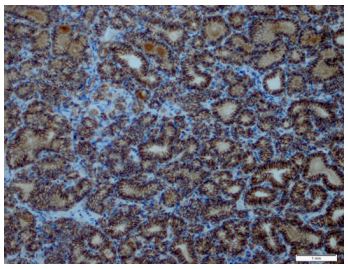
Immunohistochemical expression of CD56 showed membranous staining in lesions other than papillary carcinoma (Figure 1). In the case of follicular adenoma and Hurthle cell adenoma, cytoplasmic staining is detected (Figure 2). CD56 expression was found in >10% of cells in all cases (Figure 3). The percentage of CD56 expression was lower in follicular carcinoma and Hurthle cell carcinoma cases than in non-PTC lesions. While CD56 immunohistochemical expression was very low in cases in which we detected PTC, negative expression was detected in 8 of the cases (Figure 4). When we divide it into two as PTC and non-PTC cases, it has been determined that the expression is very low and almost non-existent in PTC cases (Figure 5). The specificity and sensitivity of CD56 immunohistochemical expression in distinguishing PTC from other follicular lesions was 95.8% and 98.6% (Figure 6).
Figures-1: Follicular variant papillary thyroid carcinoma CD56 weak expression (CD56, x200).
Figures-2: CD56-positive structures located at the interface between the tumor and the nontumor tissue or interpenetrating the CD56-negative tumor tissue (CD56, x100).
Figures-3: Positive membranous CD56 expression in follicular adenoma (CD56, x200).
Figures-4: CD56 expression in inflammatory cells and thyroid follicles in thyroiditis (CD56, x200).
Figures-5: Papillary thyroid carcinoma showed negativity for CD56 (CD56, x100).
Figures-6: Positive CD56 expression in follicular carcinoma (CD56, x200).
Discussion
CD56 is a homophilic glycoprotein molecule found in the membranes of cells [19]. CD56 is also an adhesion molecule and belongs to the immunoglobulin family [20]. CD56 is also found on the surface of neurons and skeletal muscle cells [21]. In this study, low CD56 immunohistochemical staining was detected in PTC cases, including FVPTC [20]. Thereover, it should be kept in mind that CD56 immunohistochemical expression may be positive in some cases. In our study [20], it was found that cells stained only weakly cytoplasmic and less than 10% of tumor cells were positive [21]. Thereover, positive expression was found in more than 10 percent of cells in PTC cases. Etem et al. [22] reported in their study that, contrary to our results, there was no significant differentiation in terms of CD56 staining in follicular tumor and PTC [21]. However, our study is considered positive for CD56, both cytoplasmic and membranous [22].
El Demellawy et al. [23] it showed that CD56 expression was 100% sensitive and specific in distinguishing PTC from other thyroid lesions. Ozolins et al. [24] showed that CD56 expression can be negative in follicular adenoma [24]. In addition, they reported that CD56 expression was 100% sensitive and 82% specific in the diagnosis of PTC. Park et al. [25] reported CD56 sensitivity as 92.5% and specificity as 86.4%. The results of our study, however, did not show 100% sensitivity or specificity, but confirmed that CD56 is a marker with high sensitivity and specificity [25].
Abd El Atti et al. [26] They studied the expression of CD56 and claudin-1 to differentiate between FVPCs and other follicular nodules [26]. They reported that it was 100% specific and 81.3% sensitive in this combination study [27]. In our study, the sensitivity and specificity of CD56 expression alone were 81.3% and 89.4%. Lee et al. [27] showed that CD56 expression was negative in 6 of 16 cases in a study on thyroid tumors with trabecular patterns [26,27]. And in the same study, they decided to use CK19 or Galectin-3 in 4 of 6 negative cases. CK19 and Galectin-3 are two separate markers used to differentiate PTC from other thyroid lesions. As in other studies, CD56 sensitivity and specificity were not found to be 100% in our study. Studies in the literature and our study showed that it would be useful to use markers such as CD56 when making the pathological diagnosis of thyroid diseases.
Changes in CD56 expression should not only be associated with thyroid diseases, but it should be considered that loss of CD56 expression may also occur in cases with poor prognosis in colon and pancreatic carcinomas [28] Cavallaro et al. [33] reported that CD56 mediates β1-integrin-mediated cell matrix adhesion by activating the fibroblast growth factor receptor [28]. In addition, Scarpino et al. [29] reported that CD56 expression in PTC may cause downregulation of lymphangiogenesis stimulating factors, vascular endothelial growth factor D (VEGF-D) and VEGF-C.Kim et al. [30] showed that homeobox B9 (HoxB9) regulates the transcription of CD56 in the PTC (30). Also, silencing of HoxB9 nuclear expression correlated with tumor stage and extrathyroidal spread. However, loss of CD56 expression was not associated with HoxB9 [31].
According to the results of our study; The CD56 immunohistochemical marker is both a sensitive and specific marker that can be used to differentiate PTC lesions from other follicular lesions. However, it may be better to diagnose by using it together with other combinations of markers for the pathological evaluation of patients. In addition, application of CD56 immunohistochemistry marker to cell block sections obtained from fine needle aspiration cytology may be useful for specific diagnosis. We suggest studies with larger case numbers for tumor pathological diagnosis of CD56 in PTC. Because, the number of cases was limited as no funding was received in the study.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: No
Highlights
CD56 is found in the follicular epithelial cells of the normal thyroid and shows diffuse membranous staining
It has been reported that the expression of CD56 is low or absent in PTC in immunohistochemical methods and/or analysis using
CD56 is both a sensitive and specific marker to separately distinguish PTC from all other follicular lesions of the thyroid, but for the clinical evaluation of patients it may be better to use a combination of markers.
The application of CD56 immunohistochemistry marker to cell block blocks may be useful for specific diagnosis in FNAB cases.
References
- Gharib H, Papini E. Thyroid nodules: Clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. 2007; 36: 707–35.
- Topliss D. Thyroid incidentaloma: The ignorant in pursuit of the impalpable. Clin Endocrinol (Oxf) 2004; 60: 18–20.
- Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995. Cancer. 1998; 83: 2638–48.
- Finley DJ, Arora N, Zhu B, Gallagher L, Fahey TJ. 3rd Molecular profiling distinguishes papillary carcinoma from benign thyroid nodules. J Clin Endocrinol Metab. 2004; 89: 3214–23.
- Chan J. Strict criteria should be applied in the diagnosis of encapsulated follicular variant of papillary thyroid carcinoma. Am J Clin Pathol. 2002; 117: 16–8.
- El Demellawy D, Nasr A, Alowami S. Application of CD56, P63 and CK19 immunohistochemistry in the diagnosis of papillary carcinoma of the thyroid. Diagn Pathol. 2008; 3: 5.
- Prasad ML, Pellegata NS, Huang Y, Nagaraja HN, de la Chapelle A, Kloos RT. Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol. 2005; 18: 48–57.
- Cheung CC, Ezzat S, Freeman JL, Rosen IB, Asa SL. Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod Pathol. 2001; 14: 338–42.
- Baloch ZW, LiVolsi VA. Fine-needle aspiration of thyroid nodules: Past, present, and future. Endocr Pract. 2004; 10: 234–41.
- Albores-Saavedra J, Wu J. The many faces and mimics of papillary thyroid carcinoma. Endocr Pathol. 2006; 17: 1–18.
- Schmid KW, Farid NR. How to define follicular thyroid carcinoma? Virchows Arch. 2006; 448: 385–93.
- Lloyd RV, Erickson LA, Casey MB, Lam KY, Lohse CM, Asa SL, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004; 28: 1336–40.
- Saxen E, Franssila K, Bjarnason O, Normann T, Ringertz N. Observer variation in histologic classification of thyroid cancer. Acta Pathol Microbiol Scand A. 1978; 86A: 483–6.
- Turkoz HK, Oksuz H, Yurdakul Z, Ozcan D. Galectin-3 expression in tumor progression and metastasis of papillary thyroid carcinoma. Endocr Pathol. 2008; 19: 92–6.
- Pyo JS, Sohn JH, Kang G, et al. Characteristics of neck level VI lymph nodes in papillary thyroid carcinoma: correlation between nodal characteristics and primary tumor. Endocr Pathol. 2015; 26: 15-20.
- Zeromski J, Bagnasco M, Paolieri F, Dworacki G. Expression of CD56 (NKH-1) differentiation antigen in human thyroid epithelium. Clin Exp Immunol. 1992; 89: 474–8.
- Zeromski J, Dworacki G, Jenek J, Niemir Z, Jezewska E, Jenek R, et al. Protein and mRNA expression of CD56/N-CAM on follicular epithelial cells of the human thyroid. Int J Immunopathol Pharmacol. 1999; 12: 23–30.
- Scarpino S, Di Napoli A, Melotti F, Talerico C, Cancrini A, Ruco L. Papillary carcinoma of the thyroid: Low expression of NCAM (CD56) is associated with downregulation of VEGF-D production by tumour cells. J Pathol. 2007; 212: 411–9.
- El Demellawy D, Nasr AL, Babay S, Alowami S. Diagnostic utility of CD56 immunohistochemistry in papillary carcinoma of the thyroid. Pathol Res Pract. 2009; 205: 303–9.
- LiVolsi VA, Baloch ZW. Follicular neoplasms of the thyroid: View, biases, and experiences. Adv Anat Pathol. 2004; 11: 279–87.
- Hoos A, Stojadinovic A, Singh B, Dudas ME, Leung DH, Shaha AR, et al. Clinical significance of molecular expression profiles of Hurthle cell tumors of the thyroid gland analyzed via tissue microarrays. Am J Pathol. 2002; 160: 175–83.
- Lanier LL, Testi R, Bindl J, Phillips JH. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989; 169: 2233–8.
- Zeromski J, Lawniczak M, Galbas K, Jenek R, Golusinski P. Expression of CD56/N-CAM antigen and some other adhesion molecules in various human endocrine glands. Folia Histochem Cytobiol. 1998; 36: 119–25.
- Zeromski J, Biczysko M, Stajgis P, Lawniczak M, Biczysko W. CD56(NCAM) antigen in glandular epithelium of human thyroid: Light microscopic and ultrastructural study. Folia Histochem Cytobiol. 1999; 37: 11–7.
- Park WY, Jeong SM, Lee JH, Kang HJ, Sin DH, Choi KU, et al. Diagnostic value of decreased expression of CD56 protein in papillary carcinoma of the thyroid gland. Basic Appl Pathol. 2009; 2: 63–8.
- Etem H, Özekinci S, Mizrak B, Şentürk S. The Role of CD56, HBME-1, and p63 in follicular neoplasms of the thyroid. Turk Patoloji Derg. 2010; 26: 238–42.
- Cavallaro U, Niedermeyer J, Fuxa M, et al. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol. 2001; 3: 650-657.
- Abd El Atti RM, Shash LS. Potential diagnostic utility of CD56 and claudin-1 in papillary thyroid carcinoma and solitary follicular thyroid nodules. J Egypt Natl Canc Inst. 2012; 24: 175–84.
- Lee S, Hong S, Koo JS. Immunohistochemical subclassification of thyroid tumors with a prominent hyalinizing trabecular pattern. APMIS. 2011; 119: 529–36.
- El Demellawy D, Nasr A, Alowami S. Application of CD56, P63 and CK19 immunohistochemistry in the diagnosis of papillary carcinoma of the thyroid. Diagn Pathol. 2008; 3: 5.
- Fogar P, Basso D, Pasquali C, De Paoli M, Sperti C, Roveroni G, et al. Neural cell adhesion molecule (N-CAM) in gastrointestinal neoplasias. Anticancer Res. 1997; 17: 1227–30.