Case Report - Volume 2 - Issue 6
One case of coronavirus disease 2019 (COVID-19) positive in a patient with multiple comorbidity
R Arbind Kumar Choudhary1*; Pk Sanjaikrishana2
1Assistant Professor Govt. Erode Medical college & Hospital, Perundurai, Erode -638053, India.
24th year MBBS Student, Govt. Erode Medical college & Hospital, Perundurai , Erode -638053, India.
Received Date : Sep 08, 2022
Accepted Date : Oct 25, 2022
Published Date: Nov 11, 2022
Copyright:© R Arbind Kumar Choudhary 2022
*Corresponding Author : R Arbind Kumar Choudhary, Assistant Professor Govt. Erode Medical college & Hospital, Perundurai, Erode -638053,India.Tel: 7871797278
Email:arbindkch@gmail.com
DOI: Doi.org/10.55920/2771-019X/1291
Abstract
The ongoing battle of COVID-19 India become an emergency of international concern when thousands of people were infected around the world now rapidly increasing number of case with co-morbidity is common in India . This case control study of a patient with covid 19 positive simultaneously infected by SARS-Cov-2 and multiple co morbidity. This case highlights that a co-infection of SARS-Cov-2 and multiple other elevated disease condition like type 2 diabetes mellitus / Systemic Hypertension/ coronary artery diseases/ chronic obstructive pulmonary disease, may severely impair the immune system.
Introduction
Pneumonia of unknown cause detected in Wuhan, China was first reported to the WHO Country Office in China on 31 December 2019. officially named by the World Health Organization as COVID-19, appeared in Wuhan, Hubei Province, China, WHO is working 24/7 to analyse data, provide advice, coordinate with partners, help countries prepare, increase supplies and manage expert networks. The outbreak was declared a Public Health Emergency of International Concern on 30 January 2020. On 11 February 2020, WHO announced a name for the new coronavirus disease: COVID-19. Patients present with severe viral pneumonia and respiratory illness. Lymphopenia has been considered as a poor prognostic factor for severe acute respiratory syndrome (SARS) [1] as well as COVID19 [2].
Case Presentation
A 65 year old patient known case of type 2 diabetes mellitus / Systemic Hypertension/ coronary artery diseases/ chronic obstructive pulmonary disease was admitted on 21/05/2020 with chief complaints of fever cough and breathlessness for 1 day , H/O travel from Chennai to Erode after 40 days stays during lockdown in Chennai. While evaluation of sign and symptom patient had symptom of coronavirus like fever, dry cough
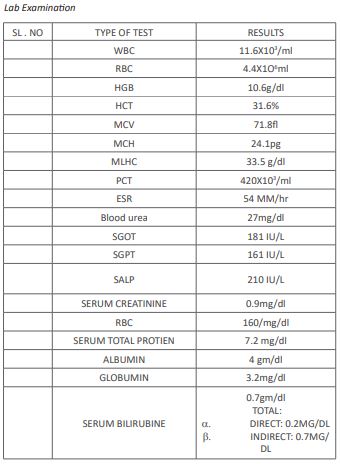
and tiredness , Initial investigation like CBC, RFT, LFT RBC, LRP, B-. UREA, SERUM CREATNINE AND CHEST X-REY were done and most important nasal and oral swab were taken.
On day one: 21/05/2020
Urine Examination:
1. YELLOW , CLEAR , ACIDIC , ABLUMINE TRAC SUGAR
+0.5%
2. BILE SALT+ -PIGMENTED
3. PUSCELLS: 6-8
4. EPITHILIAL CELLS : 1-3
5. RBC : 1-2
CHEST X -RAY Impression shown R/L lower part infiltration,
HR Chest shows
ABG shown Respiratory alkalosis
1. PH: 7.47
2. pCo2 : 22.3 mmhg
3. pO2 61mmhg
4. Na+ : 137 mmol/l
5. K+ 3.22 mmol/l
6. Ca : 0.75 mmol/l
Vitals:
1. PR-98/min
2. Rr-24/min
3. BP- 140/190 mmhg
4. SPO2 95% in room air
Impression:
1. CBC shown mild lecucytosis
2. ESR raised with higher level
3. RT-PCR report shown COVID 19 POSITIVE (+)
On the day of admission 21/05/202, patient was diagnosed as
case of SARI/hypertension /type 2DM/CAD /COPD.
Discussion
There are many coronaviruses, ranging from the common cold to much more serious viruses such as Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). They are viruses that have been transmitted from animals to people. In severe cases, coronaviruses can cause infection in the lungs (pneumonia), kidney failure and even death. At present there is no vaccine against COVID-19 [4]. In
above case patient was Nasal oxygen (4L/min) with high flow O2, drug is instituted Azithromycin 500mg OD,Inj Merophenem I.V .TDS initially, Inj methyl Predinisolone 500mg B.D given for 3 days , Inj. Insulin infusion followed by bolus regimen according random blood sugar level , patient was kept for strict glycaemic control , Inj enoxaaprine 40 u.s.c od was given for microvascular thrombosis prevention and Inj. Pantoprazole 40 mg I.V. Meanwhile Supportive measured given for B.P control by giving drugs like Diltiazem 30 mg B.D, Tab clopidogrel 75 mg (antiplatelet), Tab. Atorvastatin 10mg (hypolipidemic). Above
drug for Coronary artery disease and drugs were continued as such. Common signs are typical flu-like symptoms: a fever, cough, breathing difficulties, tiredness and muscle aches. Symptoms usually start within 3-7 days of exposure to the virus, but in some cases it has taken up to 14 days for symptoms to appear.
People of all ages can be infected. For many (more than 80% of cases), COVID-19 is mild, with minimal flu-like symptoms. Some have not shown symptoms or only very mild symptoms, more like a common cold. The majority of people who have caught the virus have not needed to be hospitalised for supportive care. However, in up to 15% of cases COVID-19 has been severe and in around 5% of cases it has led to critical
illness. The vast majority (around 98%) of people infected to date have survived [1]. we have started with Drugs for COPD: Bronchodilators, Budesonides 80 mg twice daily Levalbuterol 90mg twice daily , Ipratropium bromide (MDI) 1 ml 3-4times daily Patient was reviewed daily by physicists, anaesthetics ,
and pulmonologists. Like any other respiratory disease, COVID-19 is spread through air-droplets that are dispersed when an infected person talks, sneezes or coughs. The virus can survive from a few hours up to a few days depending on the environmental conditions. It can be spread through close contact with an infected person or by contact with air droplets in the environment (on a surface for example) and then touching the
mouth or nose (hence the common advice circulating on hand hygiene and social distancing). COVID-19 is a new coronavirus. Keep informed of the latest developments by looking out for updates and advice from your government, national diabetes association and other reliable sources [7] in the above case of comorbidity Patient improves gradually and repeat radiological examination with the help of chest x ray and CT-SCAN done shows significant decrease in lung infiltration to near normal lung parenchyma patient is comfortable , breathing difficulty below 20/min is reduced ; gradually patient improved , nasal oxygen tarpapered and stopped patient, now maintaining SP02 – 98-99% in room air temperature .On examination:
Pulse rate: 82/min
Blood pressure: 120/80 min
Respiratory rate: 20/min
SP02: 98% in room air temperature, repeatedly ABG and random blood sugar is noted
Systemic examination:
Cvs: s1-s2 heard
RS: NVBS heard, no added sound
CNS: absolutely fine
P/A: soft, not tender
Repeated RT-PCR done. RT-PCR third sample shows negative, no sign and symptom covid 19, patient successfully discharge with stable vitals.On the day of discharge 07/06/2020; random blood sugar is
105 mg/dl post prandial blood sugar level- 188mg/dl bloodsugar level found to be satisfactory.
Conclusions
Specific antiviral treatments and vaccines are still under drug development process, testing, quarantine, and social distancing are main criteria to prevent virus spread. In current scenario virus keeps mutating and evolving during the pandemic, studies on viral pathogenicity, treatments and prophylactic vaccines should monitored and proper treatment regimen along with drug of comorbidity can be managed.
References
- Leung GM, Hedley AJ, Ho LM, et al. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann Intern Med. 2004;141: 662-673.
- Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID19 in Wuhan, China. Clin Infect Dis. 2020; (accepted article). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395(10223): 497-506.
- Lee HK, Lee BH, Seok SH, et al. Production of specific antibodies against SARScoronavirus nucleocapsid protein without cross reactivity with human coronavirus. 2010; 11(2): 165-167.
- Li Z, Yi Y, Luo X, et al.Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-Cov-2 infection diagnosis. J Med Virol. 2020; (accepted article).
- C Huang, Y Wang, X Li, L Ren, J Zhao, Y Hu, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China Lancet, 2020; 395: 497-506.
- N Zhu, D Zhang, W Wang, X Li, B Yang, J Song, et al. A novel coronavirus from patients with pneumonia in China, 2019 N Engl J Med, 2020; 382: 727-733.
- F Wu, S Zhao, B Yu, YM Chen, W Wang, ZG Song, et al. A new coronavirus associated with human respiratory disease in China Nature, 2020; 579: 265-269.
- Coronaviridae Study Group of the International Committee on Taxonomy of The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2 Nat Microbiol, 2020; 5: 536-544.