Case Report - Volume 2 - Issue 6
Surgery of abdominal wall endometriosis associated with clear-cell carcinoma: Case report and review
C Petit* ; L Donval; M Chandeze; M Joste; and P Panel
Department of Gynecology and Obstetrics, Centre Hospitalier de Versailles, Le Chesnay, France.
Received Date : Sep 12, 2022
Accepted Date : Oct 25, 2022
Published Date: Nov 12, 2022
Copyright:© Petit Clémence 2022
*Corresponding Author : Petit Clémence, Department of Gynecology and Obstetrics, Centre Hospitalier de Versailles, Le Chesnay, France.
Email: lou.donval@live.fr
DOI: Doi.org/10.55920/2771-019X/1292
Abstract
Introduction: The peritoneum is a rare location for endometriosis, with a reported incidence of parietal endometriosis of approximately 0.03 to 0.4%. It most often occurs in the aftermath of a caesarean section and is associated with pelvic endometriosis in only 5 to 15% of cases. Rare cases of malignant transformation have been described, mainly in the form of clear-cell tumours.
Clinical case: We report the case of a 52-year-old female patient with a history of endometriosis who presented with a retractile parietal mass at the level of her caesarean scar. Surgical excision was performed. Histological analysis confirmed a clear-cell adenocarcinoma.
Discussion: Only about 20 cases of clear-cell carcinoma associated with parietal endometriosis have been reported. A review of the literature suggests radical surgical treatment combined with adjuvant radio-chemotherapy. However, the prognosis is still poor, with median survival of 35 months.
Conclusion: The aim of this case report is to suggest the diagnosis of malignant transformation in the presence of a rapidly evolving parietal mass in the context of endometriosis and a history of caesarean section.
Keywords: Parietal endometriosis; clear-cell carcinoma; caesarean-section scar.
Introduction
Parietal endometriosis is a rare condition with a reported incidence of 0.03% to 0.4% [1]. It occurs, on average, within three years after gynaecological surgery [2], most often following a caesarean section [3-5]. The pathophysiology of the disease is not yet well understood [6] but the main hypothesis is that it is the result of intraoperative dissemination of endometrial cells [7]. It is associated with pelvic endometriosis in only 5 to 15% of cases [3], making them two relatively distinct entities. The predominant symptomatology is cyclic catamenial scar pain. Clinical examination may reveal palpable nodules; if not, ultrasound or MRI may reveal them. When lesions are extensive and/or symptomatic, despite medical treatment, surgical management is the treatment of choice [7,8] and consists of complete removal of the nodules to limit the risk of recurrence. A prosthesis may be necessary if the size of the resection is too large. Rare cases of malignant transformation of parietal endometriotic lesions have been described [9,10]. They mainly consist of clear-cell tumours, followed by endometrioid tumours [11,12].
Here, we report the case of a patient for whom we performed the complete removal of a parietal tumour mass infiltrating the rectus muscles by more than 10 cm and then repair of the wall by the placement of a prosthesis.
Clinical case
A 52-year-old female patient consulted for pain in her caesarean scar and cyclical pelvic pain, despite amenorrhoea on continuous micro-progestogen pills. Her history included endometriosis diagnosed in 1992, resection of a parietal endometriosis nodule in 2011, a Meckel's diverticulum complicated by an occlusion treated by small bowel resection by median laparotomy in 2014, and two caesarean sections.
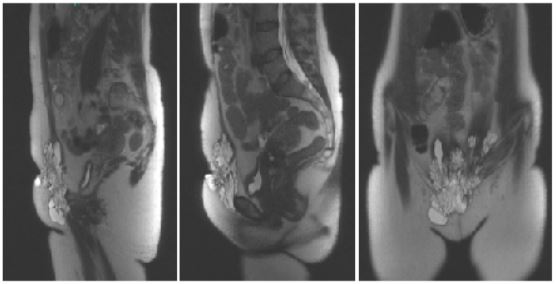
On clinical examination, there was a large bluish sub-umbilical parietal mass with skin retraction opposite the caesarean scar. The mass was hard and painful, attached to the pubis, and descended prepubically to the top of the right labia majora. Ultrasound of the soft tissues showed approximately 10 subcutaneous and subcicatricial pelvic nodular formations of 10 to 24 mm. Pelvic MRI showed a cystic parietal endometriotic nodule of 93 x 108 x 40 mm on either side of the scar (Figure 1). This lesion infiltrated the lower part of the left and right rectus muscles and extended into the right inguinal fossa and involved the medial part of the adductor muscles. The uterus was also adenomyotic and myomatous; no other endometriotic lesions were visible.
Figure 1: Pelvic MRI showing a pericicatricial parietal endometriotic nodule.
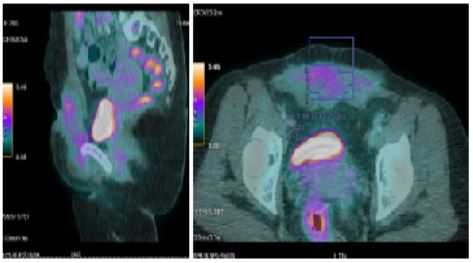
Tru-cut biopsy revealed a clear-cell tumour of which the origin and nature were difficult to determine. PET showed a heterogeneous abdominal parietal mass infiltrating the skin and muscle planes (rectus abdominis, bilateral external obliques, right pectineal muscle), with low to moderate hypermetabolism (SUVmax = 5.2), approximately 117 x 35 mm in the axial plane and 100 mm in height. There were no other distant suspicious hypermetabolic lesions (Figure 2).
Figure 2: PET scan showing a moderately hypermetabolic parietal mass.
The multidisciplinary consultation meeting recommended complete excision surgery by median laparotomy with parietal repair associated with a total conservative hysterectomy.
Surgical Procedure
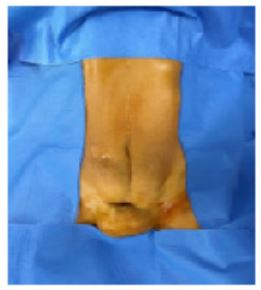
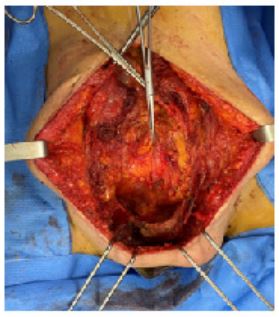
The procedure was performed under general anaesthesia with intubation. The patient was placed in the supine position. An indwelling bladder catheter was placed and surgical drapes were applied (Figure 3).
Figure 3: Large parietal mass retracting the caesarean scar.
Removal of the parietal mass
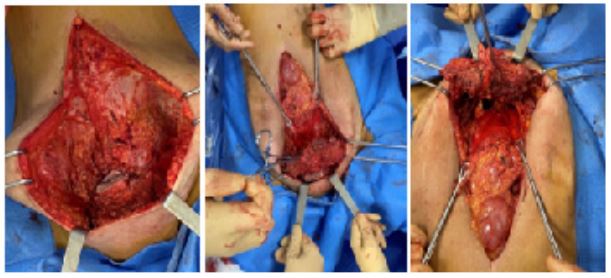
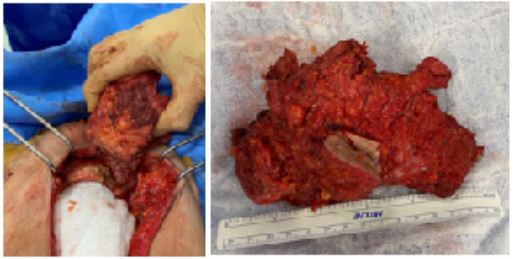
We repeated the median incision above and below the umbilical with a circular incision at the bottom, including the fistulated area of the skin. We dissected the subcutaneous plane in contact with the mass, approximately 15 cm wide and 10 cm in hight. We opened the abdominal cavity and continued the dissection by removing the rectus muscles, fascia, and anterior parietal peritoneum embedded in the mass at its lower part and the insertion of the muscles at the level of the iliopubic branches flush with the psoas on the right, with exposure of the two Cooper's ligaments, down to the labia majora (Figure 4). Dissection of the mass in contact with the pubic bone allowed complete removal of the specimen, which was sent for pathological examination (Figure 5).
Figure 4: Subcutaneous dissection of the mass carrying the fascia of the rectus muscles, with opening of the abdominal cavity.
Figure 5: Removal of the mass in contact with the pubis.
Total hysterectomy with bilateral salpingectomy
Exploration of the abdominal cavity confirmed the absence of endometriotic lesions. Total hysterectomy with bilateral salpingectomy was performed without any particularities.
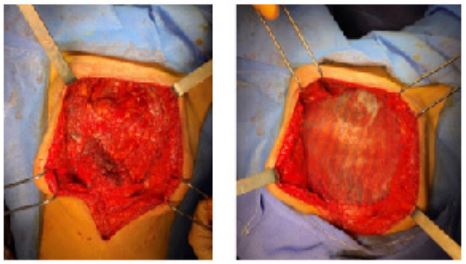
Parietal repair using a Phasix® prosthesis
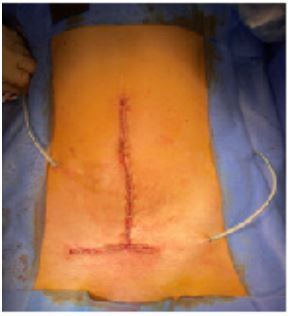
The muscle planes on either side of the defect area were detached (Figure 6). Posteriorly, there was a peritoneal plane that could be closed to isolate the prosthesis from the intra-abdominal viscera. It was not possible to create an anterior muscle plane. We decided to use a Phasix® biosynthetic prosthesis. The Phasix® prosthesis was cut into an oval shape 15 cm wide and 20 cm in hight. It was attached to the Cooper's ligament bilaterally by separate stitches of 2/0 Prolene, to the lateral fascia by separate close stitches of 2/0 Vicryl, and to the right iliac crest and fascia of the rectus abdominis by a 2/0 Vicryl overlay (Figure 7). We placed two Ch 16 redons in contact with the plate, externalized to the right and left flanks. Separate stitches of 3-0 Vicryl allowed subcutaneous approximation. We closed the skin using non-absorbable sutures and staples with an inverted T-scar (Figure 8). Abdominal restraint was provided by a pressure dressing.
Figure 6: Peritoneal plane remaining after detachment of the muscle planes on either side of the defect area.
Figure 7: Placement of a Phasix® prosthesis fixed to the fascial edges.
Figure 8: Result after skin closure, inverted T scar
Postoperative Management
The early postoperative course was simple. The patient had little pain. She was able to resume normal eating and spontaneous urination after removal of the urinary catheter on day 1 (D1). The pressure bandage was removed on D2 and the patient wore an abdominal support belt. The subcutaneous drains were removed on D6 and D7. Discharge was allowed on D7. At the postoperative consultation on D20, a subcutaneous seroma was evacuated. Local care allowed good healing. Histological analysis showed the tumour mass to be related to infiltrating clear-cell adenocarcinoma. Resection was deep with respect to the lesion and multifocal. Adjuvant chemotherapy (Carboplatin-Taxol-Avastin) was chosen. No indication for adjuvant radiotherapy was retained due to the multifocal resection margins.
Discussion
Endometriosis is a benign condition of which the proliferation and recurrence have often been compared to the development of cancerous cells. Approximately 20 cases of malignant degeneration of parietal endometriosis lesions have been described in the literature [12]. The histological type most frequently found in malignant transformation with parietal involvement are clear-cell carcinomas (63%), followed by endometrioid carcinomas (16%) and mixed tumours (21%), in contrast to ovarian tumours, in which the endometrioid type predominates [13]. To date, there are no recommendations for the management of these tumours due to the small number of cases described. A review of the literature shows mostly radical surgical treatment with complete resection of the parietal tumour, total hysterectomy, and bilateral adnexectomy, combined with adjuvant radio-chemotherapy [12-14]. Rare cases of associated lymph-node dissection have been reported, but only one of these patients had lymph-node involvement [15]. The prognosis for these patients remains poor. Furthermore, patients with clear-cell carcinomas associated with endometriosis have a poorer prognosis than those with endometrioid carcinomas, with a median survival of 35 months, despite treatment [16].
Conclusion
The incidence of parietal endometriosis, like that of pelvic endometriosis, is currently rising sharply due to improved knowledge and diagnostic methods, although they are probably two distinct entities. The potential for malignant transformation of these lesions, although rare but leading to a poor prognosis, should encourage interest in their detection and early treatment, in particular, given their comparable symptoms in the context of a previous caesarean section. The aim of this case report is to encourage gynaecologists performing endometriosis surgery to suspect malignant transformation in the presence of rapidly evolving parietal involvement.
References
- Picod G, Boulanger L, Bounoua F, Leduc F, Duval G. Endométriose pariétale sur cicatrice de césarienne : à propos de 15 cas. Gynécologie Obstétrique Fertil. janv 2006; 34(1): 8‑13.
- Horton JD, DeZee KJ, Ahnfeldt EP, Wagner M. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am J Surg. août 2008; 196(2): 207‑12.
- L’endométriose pariétale • Association EndoFrance [Internet]. Association EndoFrance. Disponible sur: https://www.endofrance.org/la-maladie-endometriose/lendometriose-parietale/
- Qu’est-ce que l’endométriose pariétale ? [Internet]. Santé.fr. 2019 [cité 1 mai 2022]. Disponible sur: https://www.sante.fr/quest-ce-que-lendometriose-parietale
- Khamechian T, Alizargar J, Mazoochi T. 5-Year data analysis of patients following abdominal wall endometrioma surgery. BMC Womens Health. déc 2014;14(1):151.
- Poismans G, Tolbize N, Gielen F, Lipombi D. Post-caesarean abdominal wall endometriosis prevention. Rev Med Liege. avr 2016; 71(4): 193‑7.
- Lamblin G, Mathevet P, Buenerd A. [Parietal endometriosis in abdominal scars. Report of 3 cases]. J Gynecol Obstet Biol Reprod (Paris). juin 1999; 28(3): 271‑4.
- Giannella L, La Marca A, Ternelli G, Menozzi G. Rectus abdominis muscle endometriosis: Case report and review of the literature: Rectus abdominis muscle endometriosis. J Obstet Gynaecol Res. 12 juill 2010; 36(4): 902‑6.
- Ferrandina G, Palluzzi E, Fanfani F, Gentileschi S, Valentini AL, Mattoli MV, et al. Endometriosis-associated clear cell carcinoma arising in caesarean section scar: a case report and review of the literature. World J Surg Oncol. déc 2016; 14(1): 300.
- Achach T, Rammeh S, Trabelsi A, Ltaief R, Ben Abdelkrim S, Mokni M, et al. Clear Cell Adenocarcinoma Arising from Abdominal Wall Endometriosis. J Oncol. 2008; 2008: 1‑3.
- Sergent F, Baron M, Le Cornec JB, Scotté M, Mace P, Marpeau L. Transformation maligne d’une endométriose pariétale. J Gynécologie Obstétrique Biol Reprod. avr 2006; 35(2): 186‑90.
- Bourdel N, Durand M, Gimbergues P, Dauplat J, Canis M. Exclusive nodal recurrence after treatment of degenerated parietal endometriosis. Fertil Steril. avr 2010; 93(6): 2074.e1-2074.e6.
- Todd RW, Kehoe S, Gearty J. A case of clear cell carcinoma arising in extragonadal endometriosis. Int J Gynecol Cancer. 3 mars 2000; 10(2): 170‑2.
- Razzouk K, Roman H, Chanavaz-Lacheray I, Scotté M, Verspyck E, Marpeau L. Mixed Clear Cell and Endometrioid Carcinoma Arising in Parietal Endometriosis. Gynecol Obstet Invest. 2007; 63(3): 140‑2.
- Primary Abdominal Wall Clear Cell Carcinoma: Case Report and Review of Literature | Anticancer Research [Internet]. [cité 24 mai 2022]. Disponible sur: https://ar.iiarjournals.org/content/29/5/1591.long
- Modesitt S. Ovarian and extraovarian endometriosis-associated cancer. Obstet Gynecol. oct 2002; 100(4): 788‑95.