Case Report - Volume 2 - Issue 6
Long-term Durable Response after Treatment with 177Lu-PSMA Therapy in Combination with Enzalutamide
Soroush Zarehparvar Moghadam1; Emran Askari2; Narjess Ayati3; Ghasemali Divband4; Kamran Aryana2*
1Nuclear Medicine Center, Velayat hospital, Qazvin University of Medical Sciences, Qazvin, Iran.
2Nuclear Medicine Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
3Department of Cancer Imaging, Peter MacCallum Cancer Center, Melbourne, VIC, Australia.
4Nuclear Medicine Center, Jam hospital, Tehran, Iran.
Received Date : Sep 16, 2022
Accepted Date : Oct 31, 2022
Published Date: Nov 19, 2022
Copyright:© Kamran Aryana 2022
*Corresponding Author : Kamran Aryana, Nuclear Medicine Research Center, Ghaem Hospital, Mashhad, Iran.
Email: Aryanak@mums.ac.ir
DOI: Doi.org/10.55920/2771-019X/1299
Abstract
A75 years-old male with metastatic castration-resistant prostate cancer (mCRPC) who had progressed under enzalutamide was treated with 4 cycles of 177Lulutetium-prostate specific membrane antigen (PSMA), as the third line of treatment for mCRPC, without discontinuing enzalutamide. The patient showed near complete response and maintained on enzalutamide for four years after PSMA-targeted radioligand therapy (PRLT) without a need for further rechallenge PRLT. Also, the patient had a long term diabetes mellitus but no nephrotoxicity regarding PRLT was observed. Long-term durable responses after PRLT without recurrence is rare and biomarkers for such phenomenon warrants further studies.
Keywords: PRLT; mCRPC; PSMA; 177Lu-PSMA; Exceptional response.
Case Report
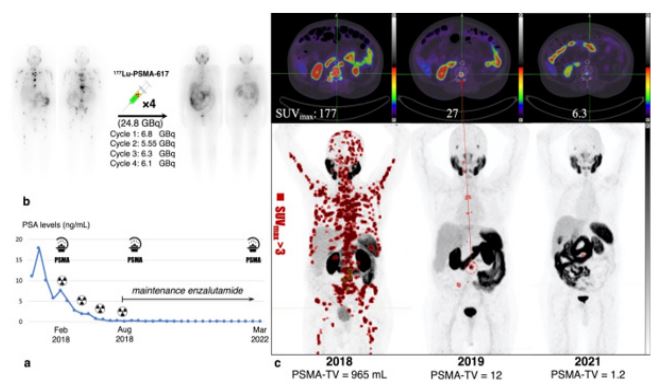
A 75 years-old metastatic castration-resistant prostate cancer(mCRPC), Gleason score = 4 + 4, initial pTNM = T3bN1M0,was referred to our center for PSMA-targeted radioligand therapy (PRLT).The patient previously received docetaxel, followed by enzalutamide and showed evidence of both biochemical and radiographic progression on these agents. His 68Ga-PSMA-11 PET/CT before PRLT showed multiple bone and lymph nodes metastasis with intense PSMA avidity while the PSA levels were 7.62ng/ml at the time of imaging. This finding was not surprising since PSA and PSMA uptake have moderate to strong association [1]. In 2018, the patient received 4 cycles of 177Lu-PSMA every 8 weeks with cumulative activity of 24.7 GBq and the PSA levels declined to 0.08 ng/ml with significant resolution of the disease burden Figure (a, b). The patient had debilitating bone pain prior to PRLT but after the first cycle of therapy the pain subsided dramatically. Transient grade Ianemia, lymphopenia and nephrotoxicity occurred during the course of PRLT. However, no severe adverse events were observed which is consistent with previous safety studies in this regard [2,3]. After finishing the 4 cycles of PRLT a near complete response was noted and the patient maintained on enzalutamide, considering the possible usefulness of this combination. Moreover, the patient had a history of long term diabetes mellitus and in a four years’ follow-up no long term nephrotoxicity and hematotoxicity was noted. The PSA levelsremainedbelow 0.1 ng/mland the patient is still alive with good quality of life and no skeletal pain. The patient showed near complete response and maintained on enzalutamide for 54 months after RLT without a need for further rechallenge RLT. In 2019 and 2021 two surveillance 68Ga-PSMA PET/CTs were performed,revealing only a small focus of PSMA-avid metastasis in the posterior element of the 3rd lumbar vertebra (SUVmax= 6.3)in the last follow up Figure (c). Previous cases with exceptional responses usually have had a relapsing course and repeated cycles of PRLT were warranted which is in contrast to our case [4]. This case illustrates an exceptional responder to PRLTwith durable response. To our knowledge, the biologically-derived markers for such phenomenon has not yet defined.
Figure (a,b) In 2018, the patient received 4 cycles of 177Lu-PSMA every 8 weeks with cumulative activity of 24.7 GBq and the PSA levels declined to 0.08 ng/ml with significant resolution of the disease burden.
c) In 2019 and 2021 two surveillance 68Ga-PSMA PET/CTs were performed,revealing only a small focus of PSMA-avid metastasis in the posterior element of the 3rd lumbar vertebra (SUVmax= 6.3)in the last follow up.
Conflict of interest: The authors had nothing to disclose.
References
- Han S, Woo S, Kim YI, et al. Concordance between Response Assessment Using Prostate-Specific Membrane Antigen PET and Serum Prostate-Specific Antigen Levels after Systemic Treatment in Patients with Metastatic Castration Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Diagnostics. 2021; 11: 663. https://doi.org/10.3390/diagnostics11040663.
- Moghadam SZ, Askari E, Divband G, et al. Efficacy, safety and prognostic factors affecting overall survival among metastatic prostate cancer patients undergoing treatment with 177Lu-PSMA-617: A single center study. Rev Esp Med Nucl Imagen Mol. 2022; 41(4): 239-246. https://doi.org/10.1016/j.remnie.2021.05.005
- Hartrampf PE, Weinzierl FX, Serfling SE, et al. Hematotoxicity and Nephrotoxicity in Prostate Cancer Patients Undergoing Radioligand Therapy with [177Lu] Lu-PSMA I&T. Cancers. 2022; 14: 647. https://doi.org/10.3390/cancers14030647
- Gafita A, Wang H, Tauber R, et al. Exceptional 4-year response to 177 Lu-PSMA radioligand therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2019; 46: 2212-2213. https://doi.org/10.1007/s00259-019-04410-8