Review Article - Volume 2 - Issue 6
Lacrimal Gland involvement in systemic Sarcoidosis
Hadi Khazaei*
Casey Eye Institute, Oregon Health & Science University, Portland, Oregon, 97239, USA.
Received Date : Sep 27, 2022
Accepted Date : Nov 02, 2022
Published Date: Nov 24, 2022
Copyright:© Hadi Khazaei 2022
*Corresponding Author : Hadi Khazaei, Casey Eye Institute, Oregon Health & Science University, Portland, Oregon, 97239, USA.
Email: khazaei@ohsu.edu
DOI: Doi.org/10.55920/2771-019X/1303
Abstract
Lacrimal gland lesions generallypresent as palpable masses in thesuperolateral aspects of the orbits. Approximately 50% of lacrimalgland masses are inflammatory lesions, 25% are lymphoid lesions or lymphoma, and the other 25% are salivary gland type tumors. Although there are overlaps and exceptions, features such as laterality, portion of gland involvement, presence or absence of bony findings, enhancement pattern, andclinical presentation are valuable in differentiating among lacrimal gland lesions. The 3D ultrasound has been used to define lacrimal gland shape, size, density, structural features the pattern of blood supply, as well as the anatomic and topographic position in the orbit. The study was conducted in the B- and 3D-modes of ultrasonography with color and energy Doppler mapping on both sides.
Keywords: Lacrimal Gland; Lacrimal Gland Imaging; Sarcoidosis.
Introduction
Sarcoidosis is an idiopathic, multisystem disorder that can affect any organ system and is mainly characterized by pulmonary, dermatologic, and ocular involvement. Its pathological hallmark is non-caseating granulomatous inflammation. Ocular involvement has been reported by different studies at a rate of 25-60% [1,2]. Although anterior uveitis is the most common manifestation of ocular sarcoidosis, any orbital structure can be involved. Lacrimal gland involvement is the most common form of orbital sarcoidosis [1,3].
Lacrimal glands are the most commonly involved structures of the orbit in orbital sarcoidosis [3,4,5]. The prevalence of lacrimal gland involvement varies across studies due to varying diagnostic criteria. Two large studies reported lacrimal gland involvement at rates of 7% and 15.8% [2,6]. Some studies based the diagnosis of orbital sarcoidosis on lacrimal gland enlargement and the presence of dry eye symptoms. However, sarcoidosis is a pathologic diagnosis, so a biopsy is recommended for a definitive diagnosis. Other relevant laboratory investigations revealed an elevated angiotensin-converting enzyme level/elevated levels of vitamin D/elevated calcium in the blood and urine.
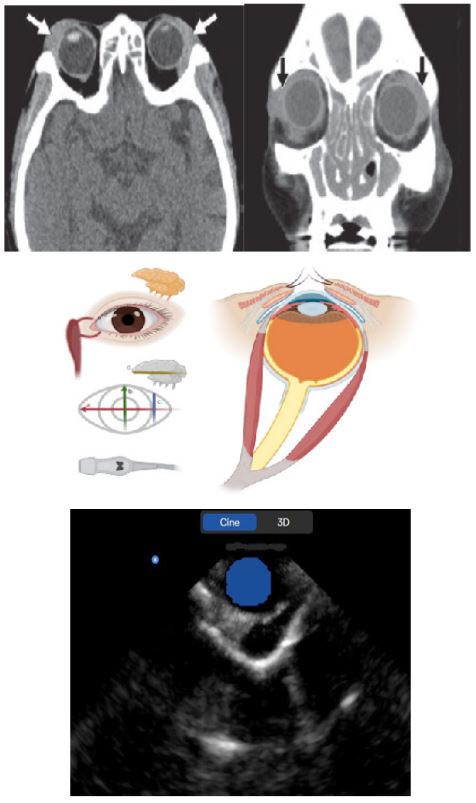
The most common imaging finding is smooth, homogenous, diffuse and nearly symmetrical enlargement of the lacrimal glands bilaterally. Although tumors and infection can cause lacrimal gland swelling, they rarely do so bilaterally. Inflammation and infiltration are much more common causes of bilateral lacrimal gland swelling with sarcoidosis, lymphoma and leukemia being the prime differentials [5]. Diffusion-weighted imaging (DWI) can potentially help in differentiating lymphomatous infiltration from sarcoidosis, with restricted diffusion and low apparent diffusion coefficient (ADC) values in the former [6,9]. Other unusual differential diagnoses also include Kimura disease and primary lacrimal amyloidosis [10,11]. However, according to Mafee et al., [12] bilateral diffuse enlargement of the lacrimal glands is highly suggestive of sarcoidosis. On imaging, normal lacrimal gland measures approximately 4-5 mm in thickness [13]. Izumi et al., [13] have shown that measuring the thickness of the lacrimal gland correlates well with measures of the areas of the lacrimal gland thus supporting the analogy that ‘size does matter’ [14].
Because of the inflammatory nature of sarcoidosis, orbital symptoms usually mimic other inflammatory diseases that involve orbital structures. Sjogren’ssyndrome, tuberculosis, lymphoma and immunoglobulin G4 (IgG4)-related Mikulicz’s disease are the main pathologies that should be considered in the differential diagnosis of sarcoidosis. Although these diseases can be seen at any age, Sjogren’s syndrome and tuberculosis are the primary diseases for the differential diagnosis of sarcoidosis in younger patients. These diseases can cause bilateral involvement and are usually characterized by painless enlargement of lacrimal glands for more than one month. Although clinical findings and imaging tests can help guide clinicians, a biopsy is required for all patients with orbital masses of unknown origin.
The characteristic histological feature of sarcoidosis is non-caseating granulomas consisting of epithelioid histiocytes and lymphocytes. Multinucleated giant cells are frequently seen. Although tuberculosis is also characterized by chronic granulomatous inflammation, in tuberculosis the granulomas tend to be coalescent with necrosis. The presence of atypical lymphocytes in lymphoma, IgG4-positive plasma cells in IgG4-related Mikulicz’s disease, periductal and perivascular inflammation of lymphocytes and intralobular fibrosis in Sjogren’s syndrome are the main factors that aid in the differential diagnosis of sarcoidosis [15].
While clinically, the heterogeneity of sarcoidosis is well characterized, the transcriptome of the sarcoidosis and underlying molecular mechanisms are not. The signal of all transcripts, small and long non-coding RNAs, can be detected using microarrays or RNA-Sequencing. Analyzing the transcriptome of tissues that are directly affected by granulomas is of great importance to understand biology of the disease and may be predictive of disease and treatment outcome.
Multiple genome wide expression studies of sarcoidosis affected tissues were published in the last 15 years. Published studies focused to examine differences in gene expression levels between sarcoidosis vs. control tissues, stable vs. progressive form of sarcoidosis, as well as sarcoidosis vs. other granulomatous and non-granulomatous diseases [16-19]. Understanding the transcriptome of tissues that are directly affected by the presence of granulomas, such as lung, skin or of fluids in the contact with granulomas such as bronchoalveolar lavage (BAL) and peripheral blood is of great importance to understand the biology of sarcoidosis. The signal of all transcripts and the majority of small and long non-coding RNA in humans can be detected by one microarray or RNA-Seq run, then submitted to analysis that allows understanding of intra- and inter-tissues gene networks that underlie the biology of sarcoidosis. Published transcriptomics studies indicate that numerous specific genes and signaling pathways implicated in inflammatory and immune T-cells and macrophage responses differentiate sarcoidosis from control tissues and other diseased tissues. Specific and common gene expressions, miRNAs and pathways were identified in peripheral blood, BAL and whole lung, orbital and lacrimal tissues lysate.
Gene expression in sarcoidosis involving the orbit or lacrimal gland can be distinguished from gene expression patterns in control tissue and overlaps with many transcripts upregulated or downregulated in the peripheral blood of patients with sarcoidosis. Rosenbaum et al. [20] analyzed gene expression profiles of unpaired lung, lymph nodes and peripheral blood from patients with sarcoidosis versus controls. In line with the previously mentioned data, the authors also noticed a strong Th1 immune response, as well as STAT1 and three STAT1 regulated chemokines (CXCL9, CXCL10 and CXCL11) to be commonly up-regulated in sarcoidosis. STAT1 was already shown to be a key inflammatory and INF-γ response transcription factor [20].
Standard operating procedure for orbital ultrasonography:
1) Obtain approved consent and authorization form
2) Clean and prepare the site of interest and ultrasound probe
3) Apply sterile coupling agents on ultrasound probe headpiece
4) Connect the probe to mobile device and launch the app
5) Select the appropriate preset to start scanning
- Horizontal linear scan (medial orientation) - adjust depth and ΔTGC
- Vertical linear scan (superior orientation) - adjust depth and ΔTGC
- Lateral vertical oblique scan (Ossining technique + doppler) - optic nerve assessment
- Horizontal linear (Lacrimal gland) scan - volumetric and ΔTGC
6) 3D scan/Cine recording of orbit
Discussion
The lacrimal gland is an almond-shaped, echini secretory gland for tear production. It is located in the superolateral aspect of theorbit, abutting the superior rectus and lateral rectus muscles. The lacrimal glandconsists of an orbital lobe and a palpebral lobe, which are separated anatomically by the lateral hornof the aponeurosis of the levator palpebrae muscle. The orbital lobe is locatedposterior and superior to the levator palpebrae aponeurosis, and the palpebral lobe is situatedanterior and inferior to it.
Infiltrative and inflammatory processes tend to have a diffuse pattern, typically involving both the orbital and palpebrallobes of the gland. On CT, the lacrimal gland is isodense to themuscle. The medial border is outlined by orbital fat and the lateral border by orbital bone. Calcifications and bony changes arewell seen on CT, and normal glandsshow symmetric contrast enhancement. Thesuperior resolution of MRI permits better assessment of the extent of glandular and periglandularinvolvement. Normal lacrimalglands have intermediate (sometimes heterogeneous) signal on both T1-weighted and T2-weighted imaging and enhance symmetricallyafter gadolinium administration [21].
Sarcoidosis is the most common inflammatorydisease involving the lacrimal gland. Ocular involvement, most commonly uveitis, occurs in 80% of patients with sarcoidosis. Sarcoidosis of the lacrimal gland is typically bilateraland diffuse, and it may cause painlessenlargement of the orbital and palpebral lobes. On CT, sarcoidosis produces diffuse bilateral lacrimal gland enlargement with hyperenhancement on contrast-enhanced images and no bone involvement. Concurrent imaging of the chest usually shows prominent mediastinal and hilarlymphadenopathy. On MRI, there is also diffuse bilateral enlargement of the lacrimalglands, which appear hypointense to isointense on T1-weighted imaging and heterogeneouslyhyperintense on T2-weighted imaging, with avid enhancement after gadolinium administration. The mainstay of treatment of sarcoidosisis corticosteroids. More aggressive forms of disease may require immunosuppressants,such as methotrexate and cyclophosphamide.
Conclusion
Orbital ultrasound (US) can demonstrate extraocular muscle reflectivity changes, orbital fat attenuation, lacrimal gland enlargements and optic nerve edema in systemic Sarcoidosis, suggesting that US is a reliable tool for the determination of disease activity. In the active phase, the extraocular muscles have a lower internal reflectivity, presumably due to edema, whereas in end-stage disease, the muscles tend to show irregular high reflectivity from the echogenic fibrotic scar tissue. Similarly, the orbital fat, lacrimal gland and optic nerve demonstrate inflammatory changes in active phase of disease which may not correlate with clinical active scoring (CAS). IN the current trial, we have investigated the efficacy and safety of orbital ultrasonography in patients with systemic sarcoidosis. The use of US has also been proposed for evaluating disease activity [22-27].
References
- Prabhakaran VC, Saeed P, Esmaeli B, Sullivan TJ, McNab A, Davis G, et al. Orbital and adnexal sarcoidosis. Arch Ophthalmol. 2007;125: 1657-1662.
- Jabs DA, Johns CJ. Ocular involvement in chronic sarcoidosis. Am J Ophthalmol. 1986; 102: 297-301.
- Mavrikakis I, Rootman J. Diverse clinical presentations of orbital sarcoid. Am J Ophthalmol. 2007; 144: 769-775.
- Demirci H, Christianson MD. Orbital and adnexal involvement in sarcoidosis: analysis of clinical features and systemic disease in 30 cases. Am J Ophthalmol. 2011; 151: 1074-1080.
- Simon EM, Zoarski GH, Rothman MI, Numaguchi Y, Zagardo MT, Mathis JM. Systemic sarcoidosis with bilateral orbital involvement: MR findings. AJNR Am J Neuroradiol. 1998; 19: 336-7.
- Srinivasan A, Dvorak R, Perni K, Rohrer S, Mukherji SK. Differentiation of benign and malignant pathology in the head and neck using 3T apparent diffusion coefficient values: Early experience. AJNR Am J Neuroradiol. 2008; 29: 40-4.
- Holzapfel K, Duetsch S, Fauser C, Eiber M, Rummeny EJ, Gaa J. Value of diffusion-weighted MR imaging in the differentiation between benign and malignant cervical lymph nodes. Eur J Radiol. 2009; 72: 381-7.
- Koşucu P, Tekinbaş C, Erol M, Sari A, Kavgaci H, Oztuna F, et al. Mediastinal lymph nodes: Assessment with diffusion-weighted MR imaging. J MagnReson Imaging. 2009; 30: 292-7.
- Sepahdari AR, Aakalu VK, Setabutr P, Shiehmorteza M, Naheedy JH, Mafee MF. Indeterminate orbital masses: Restricted diffusion at MR imaging with echo-planar diffusion-weighted imaging predicts malignancy. Radiology. 2010; 256: 554-64.
- Kodama T, Kawamoto K. Kimura's disease of the lacrimal gland. Acta Ophthalmol Scand. 1998; 76: 374-7.
- Cheng JY, Fong KS, Cheah ES, Choo CT. Lacrimal gland amyloidosis. OphthalPlastReconstr Surg. 2006; 22: 306-8.
- Mafee MF, Dorodi S, Pai E. Sarcoidosis of the eye, orbit, and central nervous system. Role of MR imaging. Radiol Clin North Am. 1999; 37: 73-87.
- Izumi M, Eguchi K, Uetani M, Nakamura H, Takagi Y, Hayashi K, et al. MR features of the lacrimal gland in Sjögren's syndrome. Am J Roentgenol. 1998; 170: 1661-6.
- Ueno H, Ariji E, Izumi M, Uetani M, Hayashi K, Nakamura T. MR imaging of the lacrimal gland. Age-related and gender-dependent changes in size and structure. Acta Radiol. 1996; 37: 714-9.
- Williamson J, Gibson AA, Wilson T, Forrester JV, Whaley K, Dick WC. Histology of the lacrimal gland in keratoconjunctivitis sicca. Br J Ophthalmol. 1973; 57: 852-858.
- Lockstone HE, Sanderson S, Kulakova N, et al. Gene set analysis of lung samples provides insight into pathogenesis of progressive, fibrotic pulmonary sarcoidosis. Am J Respir Crit Care Med. 2010. [DOI: 10.1164/rccm.200912-1855OC].
- Maertzdorf J, Weiner J, Mollenkopf HJ, et al. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc Natl Acad Sci USA. 2012; 109: 7853-8.
- Leng D, Huan C, Xie T, et al. Meta-Analysis of Genetic Programs between Idiopathic Pulmonary Fibrosis and Sarcoidosis. PLoS One. 2013; 8: e71059.
- Bloom CI, Graham CM, Berry MPR, et al. Transcriptional Blood Signatures Distinguish Pulmonary Tuberculosis, Pulmonary Sarcoidosis, Pneumonias and Lung Cancers. PLoS One. 2013. [DOI: 10.1371/journal.pone.0070630].
- Rosenbaum JT, Choi D, Wilson DJ, et al. Parallel Gene Expression Changes in Sarcoidosis Involving the Lacrimal Gland, Orbital Tissue, or Blood. JAMA Ophthalmol. 2015; 133(7): 770-777. [DOI: 10.1001/jamaophthalmol.2015.0726].
- Yiming Gao, Gul Moonis, Mary E. Cunnane,Ronald L. Eisenberg. Lacrimal Gland Masses. AJR. 2013. [DOI:10.2214/AJR.12.9553].
- Hadi Khazaei, Danesh Khazaei, Rohan Verma, John Ng, Phillip A Wilmarth, et al. The potential of tear proteomics for diagnosis and management of orbital inflammatory disorders including Graves’ ophthalmopathy, Experimental Eye Research. 2021; 213: 108813. https://doi.org/10.1016/j.exer.2021.108813.
- Khazaei H, Khazaei D, Ashraf D, Mikkilineni S, Ng JD. Overview of Orbital Ultrasonography. Ann Ophthalmol Vis Sci. 2022; 5(1): 1028.
- Khazaei H, Khazaei D, Brundage D, Mikkilineni S, Dailey RA. Facial Ultrasonography in acquired facial lipoatrophy. Inter J. Research and Scientific Innovation (IJRSI). 2021; 9: 48-51.
- Khazaei H, Khazaei D, Ashraf D, Mikkilineni S, Ng JD. Ultrasonographic Characteristics of the Facial Nerve in Patient with Bell’s Palsy. Ann Ophthalmol Vis Sci. 2022; 5(1): 1029.
- Hadi Khazaei, Alireza Mobaseri MD, Danesh Khazaei, John D Ng G. Seethapathy, MS, MRCS, FRCS Ed, FRCOphth, “ORBITAL Ultrasonography a diagnosis tool in early cellulitis” International Journal of Research and Scientific Innovation (IJRSI). 2022; 9(7): 127-130. [DOI: https://dx.doi.org/10.51244/IJRSI.2022.9711].
- Hadi M Khazaei, G Seethapathy. Time gain compensation in orbital ultrasonography. 2022; 3(3): 17-20. [DOI: https://doi.org/10.54660/Ijmabhr].