Case Report - Volume 2 - Issue 6
Abernethy Syndrome Type 1B in a Young Male presenting with acute gastrointestinal bleeding: A Case report
Dorotea Bozic1; Ivana Jukic1,*; Ante Mayer1; Liana Cambj-Sapunar2, 4; Kristian Podrug1; Tomislav Ivanovic3; Zeljko Sundov1, 4; Zeljko Puljiz1, 4; Jonatan Vukovic1,4
1Department of Gastroenterology and Hepatology, University Hospital of Split, Spinciceva 1, 21000 Split, Croatia.
2Department of Radiology, University Hospital of Split, Spinciceva 1, 21000 Split, Croatia.
3Department of Surgery, University Hospital of Split, Spinciceva 1, 21000 Split, Croatia.
4Schoolof Medicine, University of Split, Soltanska 2, 21000 Split, Croatia.
Received Date : Sep 29, 2022
Accepted Date : Nov 05, 2022
Published Date: Nov 29, 2022
Copyright:© Ivana Jukic 2022
*Corresponding Author : Ivana Jukic, Department of Gastroenterology and Hepatology, University Hospital of Split, Split, Spinciceva 1, 21000 Split, Croatia, EU. Tel: 00385 916122610
Email: ivjukic@gmail.com
DOI: Doi.org/10.55920/2771-019X/1307
Abstract
Introduction: Abernethy syndrome is a rare congenital extrahepatic portosystemic shunt (CEPS) in which splanchnic blood flows straightly into the inferior vena cava (IVC). Compared to type 1, in which the intra hepatic portal tree is not developed, in type 2 the portal vein is intact, but forms an anastomosis with the IVC. In the subtype 1a, superior mesenteric and splenic veins do not merge into the portal vein, while in type 1b they anatomically form the portal vein. CEPS can cause a wide spectrum of clinical manifestations including hepatic, pulmonary, metabolic and neurological symptoms and is usually diagnosed before adulthood. The diagnosis is established using the radiological imaging methods. Whileliver transplantation is the treatment of choice for type 1, surgical or minimally invasive endovascular shunt closure are treatment options for type 2 Abernethy syndome.
Case report: We are reporting a case of a 34-year-old male Caucasian patient that was admitted to the department of gastroenterology and hepatology due to gastrointestinal bleeding from the Forrest Ib gastric ulcer. Diagnostic work-up revealed fibrosis of the liver as well as the existence of a congenital portocaval shunt without formed intrahepatic portal branches. He was diagnosed with the Abernethy syndrome type 1b. The anatomy of the hepatic veins was also abnormal, since they were shrinking towards the hilus of the liver and drained from there into the superior vena cava. Laboratory parameters revealed slight impairment of liver function and high ammonia levels with covert hepatic encephalopathy. The patient is currently under conservative treatment and strict follow-up, as a potential candidate for liver transplantation management.
Conclusion: Altough a rarecondition, Abernethy syndrome must be included in the differential diagnosis of patients presenting with hepato pulmonary sindrom, hypergalactosemia, gastrointestinal bleeding and hyperammonemia. The patients should be given the adequate treatment determined on individual basis and initiated with the appropriate timing.
Keywords: Abernethysyndrome; congenitalextrahepatic porto-systemicshunt; gastrointestinalbleeding; hyperammonemia.
Introduction
Abernethy syndrome was first described by John Abernethy in 1793. As a congenital extra hepatic portosystemic shunt (CEPS), when splanchnic blood flows straightly into the inferior vena cava (IVC) [1]. Extra hepatic shunts are commonly accompanied by other congenital anomalies and are further divided according to Morgan and Super in a into three types [2]. Type 1 represents complete shunting of the portal venous blood (i.e., end-to-side shunt) with severe hypoplasiaor total absence of the intra hepatic tree. In CEPS type 2 portal vein isintact, but a side-to-side anastomosis with the IVC leads to shunting [3]. Type 1 can futher be divided into sub types 1a and 1b. In the sub type 1a, superior mesenteric vein and the splenic vein do not connect and thus there is no anatomical portal vein. Contrary, inthe 1b subtype, the aforementioned veins connect to form a portal vein [4]. The estimated incidence is around 1 in 30,000 births [5]. Clinical manifestations are extremely variable, involving hepatic, pulmonary, metabolic and neurological symptomatology. Diagnosis of Abernethy syndrome is usually made using the Doppler ultrasonography of the liver, or using other radiological imaging methods [6].
Case Report
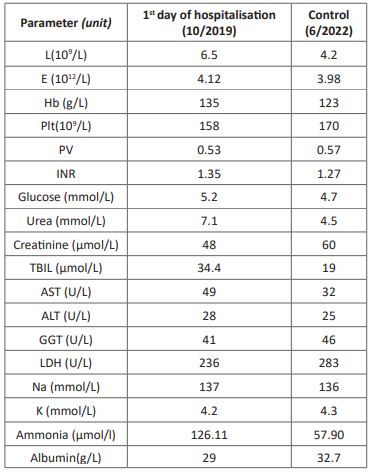
We are reporting a caseof a 34-year-old male Caucasianpatientwiththe CEPS type Ib. Besides heart murmur in childhood, he had no past medical history and was not taking any medications. In October 2019 he was admitted to the Intensive care unit of the Department of gastroenterology and hepatology due to melaena. Physical examination revealed a blood pressure of 115/65 mmhg, and a heart rate of 80 beats per minute, without any pathological signs in the clinical status. Initial laboratory work up revealed a normal red blood cell and plateletcount, but the coagulation and biochemistry panel pointed to abnormal liver function and high ammonia levels (Table 1). He under went gastroscopy that revealed venectasiae in the distal part of the esophagus as well as active bleeding due to ulceration in the proximal part of the corpus (Forrest Ib). Successful mechanical hemostasis was achieved and the control gastroscopy showed ulcer healing. Multiple bioptic sample of the ulcer borders taken for pathohistological analysis revealed only chronic inflammation of gastric mucosa, without the H. Pylori colonisation. Colonoscopy revealed the hemorrhoidal disease. Abdominal ultra sound reported normal-size dliver with diffusely inhomogenous liver parenchyma with regular contours. Two dimensional shear wave liver elastography (2D SWE) was performed (Toshiba Aplio i500, Canon) and revealed mean liver stiffness of 9,9k Pa with the inter quartile range (IQR) of 1,8. The hepatic artery wasdilated, with 7 mm of diameter and regular spectral view, with the peak systolic velocity (PSV) of 60 cm/s, and normal resistive index (RI) of 0,59. The portal vein was dilated, without vissible intra hepatic branches, flowing directly into the IVC. A lienal vein was also dilated and tortuous (12 mm). Spleen measured 14cm with formed perisplenic collaterals. There was no free liquid in the abdomen or abnormality of other parenchymal organs. Ultrasound and color doppler images are presenteding (Figure 1).
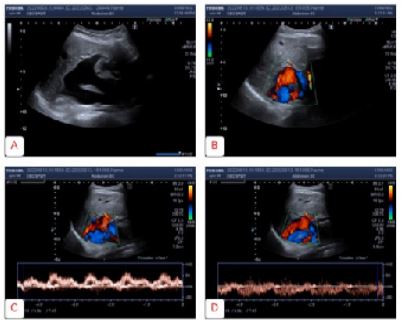
Figure 1: Ultrasound and Color Doppler imaging: Portocaval shunt presented in B mode (A) and using the Color Doppler imaging (B). Pulse wave doppler of the blood flow in the portal (C) and caval part of the shunt (D).
Table 1: Standard laboratory panel.
L: Leukocytes, E: Erythrocytes, Hb: Hemoglobin, Plt: Platelets, PV: Prothrombin Time, INR: International Ratio, TBIL: Total Bilirubin, AST: Aspartate Transaminase, ALT: Alanine Transaminase, GGT: Gamma-Glutamyl Transferase, LDH: Lactate Dehydrogenase, Na: Sodium, K: Potassium.
Sonographic findings were consistent with the F3 liver fibrosis and the suspected presence of a portocaval shunt. A thorough medical history and laboratory examination was performed and revealed no signs of viral, toxic, metabolic or auto immune liver disease (Table 2). Magnetic resonance imaging (MRI) and the contrast-enhanced multisliced computed tomography (MSCT), as well as the digital subtraction angiography (DSA) and the cavography were performed and confirmed the existence of the congenital portocaval shunt without formed portal branches, as well as the varicose veins that would indicate forming of the collateral path ways (Figure 2 and 3). The anatomy of the hepatic veins was also abnormal, since they were shrin king towards the hilusoftheliver and drained in to the superior vena cava. Theiliac and renalveins had hemodynamically better drainage towards the superior vena cava. This explained the expansion of the mesenteric venous branches and the lienal vein resistance when flowing into the shunt. Echocardiography showed trivial mitral and aortali nsufficiency and slightly dilated thoracic aorta.
He wa treated with the proton pump inhibitors (PPI), lactulose, peroral metronidazole, Lornithine L-aspartate, infusions of crystalloids and blood transfusions. Through out the hospitalization the patient was hemodynamically stable, without subjective discomfort, and after the clinical improvement he was discharged for further ambulatory follow-up and alsosent for the further evaluation to the national transplant center. Due to patients height, morphological characteristics and echocardiographically described slightly dilated thoracic aorta, we decided to perform genetic testing to exclude the Marfan syndome. A next Generation gene sequencing of the Ehlers-Danlos panel was performed at the University of Tartu, Institute of Genomics Core Facility and it exclude dany genetic mutations from the spectrum of the here ditary connective tissue diseases. He is currently being treated with lactulose and L-ornithine L-aspartate due to continously high ammonia levels and currently refuses to be included on the national list for liver transplantation.
His last ambulatory checkup was in June 2022., and hisl aboratory parameters showed a slight improvement of the liver function with significantly lower ammonia levels (Table 1).
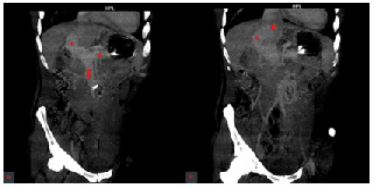
Figure 2: Portocaval shunt on the MSCT: coronal images (A and B)
MSCT: multislice computer tomography; red star:portal vein, red triangle:splenic vein, red arrow:superior mesenteric vein, red circle:superior vena cava.
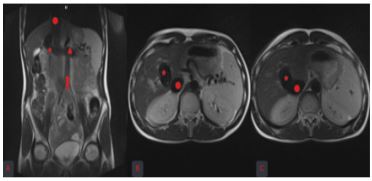
Figure 3: Portocaval shunt on the MRI: coronal (A) and axial images (B and C)
MRI: magnetic resonance imaging; red star:portal vein, red triangle:splenic vein, red arrow:superior mesenteric vein, red circle:superior vena cava.
Figure 2: Algorithm for classifying hip status.
Discussion
Clinical presentation
Since 1793, when John Abernethy first described a case of a young girl with various congenital abormalities including the portosystemic shunt, approximately 310 cases have been described in the literature until this decade [7]. While type 1 CEPS is more common in females, presents early in life, and is usually associated with other congenital anomalies such as polysplenia, cardiac defects, genitourinary diseases, skeletal abonormalities, intestinal malrotation and biliary atresia, type 2 is more common in men and might present later in adult life, similar as our patient that presented in his early thirties [8,9]. CEPS can cause a wide spectrum of clinical manifestations, including the hepatopulmonary syndrome (HPS), hepatorenal syndrome, gastrointestinal bleeding (GIB), as well as neurological symptoms due to metabolic derangements such as hyperammonemia, hypoglycemia and hyper galactosemia [10]. It might present and be diagnosed in any age, from prenatal phase to adulthood, but up to 70% of cases are diagnosed before the maturity phase [7].
Gong etal. Described six patients that presented with the lower GIB due to rectal and colonic varices, caused by an extra hepatic shunt that drained portal blood into the iliac vein via the inferior mesenteric vein. According to literature, shunts that end at the iliac veins are the most common cause of GIB inpatientswith CEPS [11]. Our patient bled from the ulceration located in the proximal part of the corpus, and showed no endoscopic signs of oesophageal or gastric varices.
HPS is caused by pulmonary vascular dilatation due to alterations in metabolism of substances that avoid passing through the liver regarding the shunt [8]. In other words, humoral factors such as endothelin, angiotensin and bacterial endotoxins, that are normally present in splanchic venous circulation and metabolised in the liver, are in CEPS redirected into the IVC and further into pulmonary circulation causing vascular dilatation, right-left shunting and hypoxemia [12]. Our patient had normal oxygen saturation, without dyspnea or other respiratory symptoms, as well as normal echocardiography with trivialmitral and aortal insufficiency and insignificant dilatation of thoracic aorta. Regarding hyper galactosemia, massive neonate screening for aforementioned metabolic derangement led to early recognition and consequently the highest prevalence of CEPS in Japan [13]. It is estimated that hyper galactosemia is present in up to 70% of newborns with CEPS, because galactose by passes the liver in the presence of shunt [5,14,15].
Hyperammonemia causing hepatic encephalopathy is usually mild, possibly as the result of adaptive mechanisms throught life. Overt encephalopathy is present in about 10% of patients, mainly in the elderly, or when the shunt ratio exceeds 60%. It occurs more commonly in patients with type 1 than in type 2 (50 % vs 22%) [7]. Kim et al compared clinical characteristics of patients treated for Abernethy syndrome in Korea, China and France. The most common initial symptom in Korea and China was GIB, while in France patients usually presented with dyspnea and central nervous system (CNS) symptoms [9,11,16]. The largest observational, multicenter, international study that included 66 patients with CEPS was published in 2020 and revealed the most common clinical characteristics:19 patients presented with he, 8 patients presented with pulmonary artery hypertension, and two with the HPS [5]. Our patient had covert hepatic encephalopathy due to long standing adaptive mechanisms on hyper ammonemia that persisted even after introduction of conservative treatment.
CEPS is commonly accompanied with the liver mass lesions such as focal nodular hyperplasia, nodular regenerative hyperplasia, liver hemangioma and adenoma, hepatoblastoma, and hepatocellular cancer (HCC), which are probably caused by decreased perfusion of hepatic tissue with portal blood and concomitantly increased hepatic arterial blood flow [10, 15]. The earliest HCC case in CEPS described in literature was a 12-month-old infant that underwent liver donor liver transplantation (LDLT) [10]. Our patient underwent several imaging methods that did not detect any focal lesions in the liver parenchyma.
Diagnosis
In patients with clinical presentation of hepatic encephalopathy or gastrointestinal bleeding abdominal ultrasound is commonly included in the diagnostic algorithm. Often, CEPS is detected accidentally in asymptomatic patients, or contrary, as a part of wide diagnostic panel while putting the puzzles of patient’s symptoms into a framed picture. During the sonographic evaluation of the liver, examiner must be careful not to miss liver nodules and enable their adequate characterisation using contrast enhanced ultrasound (CEUS) or additional radiologic methods, even in the youngest of patients. Color Doppler reveals the portocaval shunt and helps visualize whether intrahepatic portal vein branches are present and thus makes a distinction between CEPS type 1 and 2.
Our patient had a normally sized liver, with the diffusely inhomogenous liver parenchyma, and no intrahepatic portal vein branches, with the shunt visible both in the B mode and with the Color Doppler mode. Using the Pulse Wave Doppler (PW) we found a nonspecific arterialized and undulating hepatoportal flow in the PV entering the shunt, with the mirrored PW picture of flow in the IVC exiting the shunt. Hepatic artery was wide, hypertrophic, with the normal spectral view and normal RI (Figure 1). The hepatic artery dilatation is common in patients with CEPS as a compensation for the lack of portal blood flow [1].
Liver ultrasound is usually complemented with other radiologic methods including abdominal MSCT, liver MR and angiography. The MSCT angiography in our patients additionally found an abnormal anatomy of hepatic veins that were shrinking towards the hilusof the liver and drained into the superior vena cava. None the less, sometimes a live rbiopsyis needed to confirm or exclude the presence of venules in the portal triads, which is important for distinguishing between type 1 and 2 ECPS [2,13].
Treatment
Treatment strategy is individual and depends on the shunt type and the severity of clinical presentation. In the type 1 CEPS, the shunt represents the only drainage route for the mesenteric bloodand the shunt occlusion is thereforenot a treatment option. Thus, the treatment of choice is liver transplantation (LT). Apart from the LT using the graft from the dead donor, LT may be performed using the left liver lobe from the live donor (LDLT), which is commonly seen when children are affected [10,17]. Portal vein reconstruction can be difficult and require venous interposition graft due to the short portal conduit [7,17]. Auxiliary partial orthotopic liver transplantation (APOLT) is also a possible treatment option, because portal vein diversion is not necessary, and it seems technically feasible if the anatomy is appropriate [18,19]. Our patient was sent to the National transplant center, where he was proposed to be enlisted on the Croatian transplant list under the Euro transplant International Foundation. Due to his good clinical condition, patient refused LT.
In the CEPS type 2, treatment of choice is shunt occlusion which leads to redirection of blood flow and opening of hypo plastic portal veins. Shunt closure can be performed by surgical ligation or using the endovascular methods. Endovascular treatment is minimally invasive option that has demonstrated excellent results. Several materials are being used for shunt occlusion, including stents, coils, Amplatzer vascular plugs, n-butyl cyanoacrylate, as well as atrial septal defect (ASD) and ventricular septal defect (VSD) occlude devices. According to studies, if it is possible to perform the shunt closure satisfactory, it is recommended to carry out the procedure before the significant complications occur [9]. Prophylactic anticoagulationis suggested by certain authors, inorder to prevent thrombosis following shunt closure [5]. However, shunt closure may lead to certain complications, such as acute increase of portal pressure leading to spleen rupture or GIB due to failure of intrahepatic portal system expansion after shunt closure. Also, migration of coils or plugs might occur and cause obstructionof a systemic vessel. Therefore, it is recommended to estimate the portal pressure after temporary shunt closure and according to the pressure decide whether to perform the complete closure in one act or in two or three stages [7,19,20]. Rajeswaran et al. Compared surgical and endovascular treatment and found significantly lower portal pressure gradients for endovascular compared to surgical closure [19].
Conclusion
Altough a rarecondition, CEPS must be included in the differential diagnosis of patients presenting with hepatopulmonary sindrom, hypergalactosemia, gastrointestinalbleeding and hyperammonemia. Adequate diagnostic testing using various radiologic methods, as well as exclusion of other congenital anomalies should be performed.The patients should begiven the adequate treatment determined on individual basis and initiated with the appropriate timing
References
- Kumar A, Kumar J, Aggarwal R, Srivastava S. Abernethymal formation with portal vein aneurysm. DiagnInterv Radiol. 2008; 14: 143-6.
- Bernard O, Franchi-Abella S, Branchereau S, Pariente D, Gauthier F, et al. Congenital porto systemic shunts in children: recognition, evaluation, and management. Seminars in liver disease. 2012; 32(4): 273-287.
- Morgan G, Superina R. Congenital absence of the portal vein: two cases and a proposed classification system for porta systemic vascular anomalies. J Pediatr Surg. 1994; 29: 1239-41.
- Niwa T, Aida N, Tachibana K, Shinkai M, Ohhama Y, Fujita K, et al. Congenital absence of the portal vein: clinical and radiologic findings. Journal of computer assisted tomography. 2002; 26(5): 681-686.
- Baiges A, Turon F, Simón-Talero M, Tasayco S, Bueno J, Zekrini K, Plessier A, et al. REHEVASC, VALDIG an EASL consortium, Abernethy group. Congenital Extra hepatic Porto systemic Shunts (AbernethyMalformation): An International Observational Study. Hepatology. 2020; 71(2): 658-669. [DOI: 10.1002/hep.30817].
- De Gaetano AM, Rinaldi P, Barbaro B, Mirk P, Di Stasi C, Gui B, et al. Intra hepatic porto systemic venous shunts: Color Doppler sonography. Abdom Imaging. 2007; 32: 463-9.
- Ponziani FR, Faccia M, Zocco MA, Giannelli V, Pellicelli A, Ettorre GM, et al. Congenital extra hepatic porto systemic shunt: description of four cases and review of the literature. Journal of ultrasound. 2019; 22(3): 349-358. https://doi.org/10.1007/s40477-018-0329-y
- Alvarez AE, Ribeiro AF, Hessel G, Baracat J, Ribeiro JD. Abernethymal formation: one of the etiologies of hepato pulmonary syndrome. Pediatric pulmonology. 2002; 34(5): 391-394. https://doi.org/10.1002/ppul.10182
- Kim ES, Lee KW, Choe YH. The Characteristics and Outcomes of Abernethy Syndromein Korean Children: A Single Center Study. Pediatric gastroenterology, hepatology&nutrition. 2019; 22(1): 80-85. https://doi.org/10.5223/pghn.2019.22.1.80
- Benedict M, Rodriguez-Davalos M, Emre S, Walther Z, Morotti R. Congenital Extra hepatic Porto systemic Shunt (Abernethy Malformation Type Ib) with Associated Hepato cellular Carcinoma: Case Report and Literature Review. Pediatric and developmental pathology: the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2017; 20(4): 354-362. https://doi.org/10.1177/1093526616686458
- Gong Y, Zhu H, Chen J, Chen Q, Ji M, Pa M, et al. Congenital porto systemic shunts with and without gastro intestinal bleeding - case series. Pediatric radiology. 2015; 45(13): 1964-1971. https://doi.org/10.1007/s00247-015-3417-6
- Sze DY, Berquist WE. SIR 2008 annual meeting film panel case: Abernethy malformation. Journal of vascular and inter ventional radiology: JVIR. 2008; 19(9): 1274-1277. https://doi.org/10.1016/j.jvir.2008.04.017
- Turon F, Simón-Talero M, Tasayco S, Bueno J, Zekrini K, Plessier A, et al. REHEVASC, VALDIG an EASL consortium, Abernethy group. Congenital Extra hepatic Porto systemic Shunts (Abernethy Malformation): An International Observational Study. Hepatology. 2020; 71: 658-69.
- Sokollik C, Bandsma RH, Gana JC, van den Heuvel M, Ling SC. Congenital porto systemic shunt: characterization of a multi system disease. J Pediatr Gastroenterol Nutr. 2013; 56: 675-81.
- Peček J, Fister P, Homan M. Abernethy syndrome in Slovenian children: Five case reports and review of literature. World J Gastroenterol. 2020; 26(37): 5731-5744. [DOI: 10.3748/wjg.v26.i37.5731].
- Franchi-Abella S, Branchereau S, Lambert V, Fabre M, Steimberg C, Losay J, et al. Complications of congenital porto systemic shunts in children: the rapeutic options and outcomes. J Pediatr Gastroenterol Nutr. 2010; 51: 322-30.
- Nam HD. Living-donor livertransplantation for Abernethymalformation - casereport and reviewof literature. Annalsofhepato-biliary-pancreaticsurgery. 2020; 24(2): 203-208. https://doi.org/10.14701/ahbps
- Ringe KI, Galanski M, Ringe B. From Abernethy to APOLT. Liver Transpl. 2008; 14(7): 1067-8. [DOI: 10.1002/lt.21457].
- Bombardier B, Alli A, Rohr A, Collins Z, Raval K. A case of two shunts in the endovascular treatment of type II Abernethy syndrome. CVIR endovascular. 2022; 5(1): 3. https://doi.org/10.1186/s42155-021-00279-7
- Rajeswaran S, Johnston A, Green J, et al. Abernethy Malformations: Evaluation and Management of Congenital Portosystemic Shunts. J VascInterv Radiol. 2020; 31(5): 788-794. https://doi.org/10.1016/j.jvir.2019.08.

