Case Report - Volume 2 - Issue 6
Pediatric awake ECMO: A First Successful Case Report in a Portuguese Intensive Care Unit
Inês Rosinha1*; Marta João Silva1,2; Cláudia Silva1,2; Marta Grilo1,2; Carolina Baptista1; Lurdes Lisboa1; Augusto Ribeiro1
1Pediatric Intensive Care Medicine Department, São João Hospital, Portugal.
2Pediatrics Department, Oporto Medical School, Portugal.
Received Date : Sep 30, 2022
Accepted Date : Nov 08, 2022
Published Date: Dec 01, 2022
Copyright:© Inês Rosinha 2022
*Corresponding Author : Inês Rosinha, Pediatric Intensive Care Medicine Department, São João Hospital, Alameda Professor Hernâni Monteiro, 4200-319 Portugal.Tel: +351911778069
Email: inesmendrosinha@gmail.com
DOI: Doi.org/10.55920/2771-019X/1309
Abstract
Extracorporeal membrane oxygenation is a lifesaving modality of mechanical support in patients with refractorysevere acute respiratory distress syndrome, among other indications. Despite the still limited experience, the practice of “awake” extracorporeal membrane oxygenation has merged as an option for respiratory management with several advantages like de-escalation of pharmacologic sedation. A 5-year-old girl with history of asthma was admitted because of status asthmaticus secondary to Rhinovirus infection. Pulmonary auscultation revealed an overall decrease in vesicular murmur with wheezing and patient’s chest radiograph demonstrated diffuse bilateral coalescent opacities.
Despite medical treatment, her condition deteriorated rapidly, prompting transfer to the pediatric intensive care unit and initiation of non-invasive mechanical ventilation. In the next few hours, subcutaneous emphysema and hypoxic acute respiratory failure were evident and invasive mechanical ventilation was assured. Due to refractory hipoxemia with worsening of the air leak, she was placed on venovenous extracorporeal membrane oxygenation on day 2 post admission. There were no related complications and she started showing gradual clinical improvement, making extubation to humidified high-flow nasal cannula possible on day 5. She remained in humidified high-flow nasal cannula under minimal sedation, showing an exquisite level of collaborationfor her age. After 3 days, the patient was successfully decannulated and complete weaning off respiratory support was achieved 2 days later.
This isthe first attempt of pediatric “awake” ECMO in our Pediatric Intensive Care Unit, and, to our knowledge, this is also the first Portuguese report of a well-succeeded extracorporeal membrane oxygenation procedure in a minimally sedated, non-intubated pediatric patient. Our report corroborates the actual evidence that, in selected patients, extubation and appropriately decreased sedation is safe and does not compromise extracorporeal life support effectiveness. Therefore, awake extracorporeal membrane oxygenation is likely to become the new standard of care for selected pediatric patients with acute respiratory distress syndrome.
Keywords: Asthma; Acute Respiratory Distress Syndrome; Venovenous Extracorporeal Membrane Oxygenation; Mechanical Ventilation; Rhinovirus; Sedation.
Introduction
Extracorporeal membrane oxygenation (ECMO) is a lifesaving technique designed to provide mechanicalsupport to the heart, lungs or both [1-4]. ECMO has emerged as a global standard of care in potentially reversible causes of cardiopulmonary collapse or as a bridge to heart and/or lung transplantation [1-4]. Due to favorable results and a steady decline in absolute contraindications, its use is increasing worldwide, but overall survival for pediatric ECMO has remained stable around 50 to 60% [1,5]. The principle of ECMO consists in taking the deoxygenated blood from the right heart, entering the ECMO circuit, passing through the membrane lung where oxygenation, carbon dioxide clearance and temperature control are provided, and finally returning to either the arterial system (venoarterial) or the right heart (venovenous, VV-ECMO) [6].
Asthma is the most common respiratory disorder of childhood and even though multiple triggers have already been identified, infections are still the current leading cause [7-9]. Rhinovirus (RV) is a common pathogen, causing up to 60% of pediatric asthma-associated hospitalizations [7,8]. Previous studies have even stated that asthmatic children with RV infection have higher rates of emergency department visits, hospitalizations and need for escalated respiratory support [7,8]. VV-ECMO remainsan effective and widely used rescue therapy for pediatric respiratory failure refractory to conventional management, allowing time for recovery from the acute hypoxic insult and treatment of the primary disease [2,5,9-14].
Historically and in current practice, patients are intubated prior to cannulation and pediatric ECMO has traditionally been performed on sedated and intubated patients, due to the risk of life-threatening complications like dislocation of the cannula, bleeding or unintentional decannulation [15]. Furthermore, during the period of ECMO, pediatric patients usually need large doses of sedation and analgesia, which has showed a strongcorrelation with delirium, prolonged hospital stay and poor prognosis [2].
Within the last decade, the concept of awake ECMO was introduced to avoid the potential complications inherent to long-term ventilation and general sedation [15,16]. It consists in patients either not beingintubated during cannulation or being extubated soon after cannulation, while still on ECMO [15,16]. Despite the still limited experience in pediatric age, the practice of awake ECMO in extubated patients with respiratory failure has merged as an option for respiratory management with several advantages over mechanical ventilation [13,17]. Herein we present the first report ofsuccessful trial of pediatric awake ECMO in our Pediatric Intensive Care Unit (PICU).
Case Report
A 5-year-old girl with history of asthma presented to the emergency department complaining about three days of cough, breathing difficulty, decreased appetite and recent onset fever (39ºC). There was no background of Coronavirus Disease 2019 (COVID-19) infection and she had an updated vaccination plan with no immunization against COVID-19. At physical examination, she showed tachypnea (55 cycles per minute) and respiratory effort, with peripheral oxygen saturation of 90% (inspired fractional oxygen - FiO2 0.21). Pulmonary auscultation revealed an overall decrease in vesicular murmur with scattered wheezing and patient’s chest radiograph demonstrated diffuse bilateral coalescent opacities (figure 1.A).Notable labs included hemoglobin 13.2 g/dL, leukocyte count 11590/uL (68% neutrophils and 20% lymphocytes) and C-reactive protein 6.2 mg/L.
Rhinovirus was isolated in nasopharyngeal secretions. Corticosteroid, antibiotic and inhalation therapies were provided.Despite medical treatment and oxygen therapy by high flow mask at 15 liters per minute, her condition deteriorated rapidly, and prompting transfer to the PICU. She was initially placed on non-invasive mechanical ventilation (MV), but subcutaneous emphysema and pneumopericardium (Figure 1.B) arose in the following hours, associated with aggravated hypoxic acute respiratory failure, and so invasive MV was provided. In arterial blood gas (prior to intubation, FiO2: 1.0): pH 7.23, pCO2 45.3 mmHg, pO2 107 mmHg, bicarbonate 18.6 mmol/L and lactate 1.3 mmol/L.
Over the course of the next few hours, her oxygenation index remained increased (12.7) and she maintained refractory hypoxemia with worsening of the air leak. Therefore, she was cannulated with a 13-French venous cannula (Medtronic®) in the right internal jugular vein and a 19-French arterial cannula (Maquet®) in the left femoral vein (HLE 5.0 Maquet® circuit) on day 2 post admission. No complications arose during or after the procedure and then the patient started showing gradual clinical improvement. The patient was extubated to humidified high-flow nasal cannula (HFNC) on ECMO day 5, when the air leak was almost resolved (Figure 1.E), maintaining respiratory support on ECMO flow of 132-137 mL/kg/min, sweep gas flow of 0.9-2.0 L/min and FiO2of 1.0.
In HFNC, she underwent only minimal sedation with morphine at 0.02 mg/kg/hour for comfort and prevention of excessive mobilization. She was extremely cooperative and there were no cannula or circuit-related complications. After 3 days (on ECMO day 8), the patient was successfully weaned from ECMO and tolerated progressive enteral feeding. Two days post decannulation she was completely weaned off respiratory supportwith HFNC.
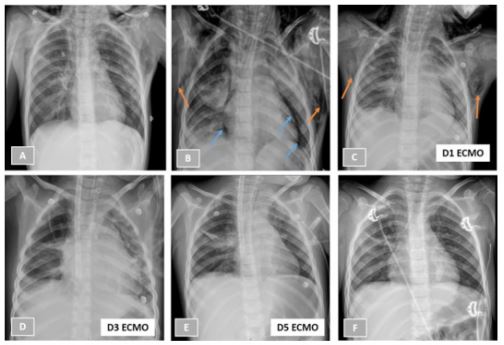
Figure 1: Patient’s chest radiographs on hospital admission (1.A), under non-invasive mechanical ventilation (1.B), on days 1, 3, 5 of extracorporeal membrane oxygenation (1.C to 1.E) and after decannulation (1.F). Orange arrows are showing subcutaneous emphysema (1.B and 1.C) and blue arrows locate pneumopericardium (1.B).
Discussion
Over the past few years, there have been steady advancements in the field of pediatric ECMO [12]. VV-ECMO provides only support for gas exchange with blood returning to the central venous circulation with no direct contribution to the systemic arterial circulation [11]. ECMO itself does not heal or treat the underlying condition(s), but it provides cardiac and/or respiratory support to allow the pathophysiology to resolve without toxic ventilation and/or inotropic support [12].
In the past, pulmonary parenchymal disease states constituted the main indication for respiratory ECMO, but nowadays it has been increasingly used to support children with severe respiratory failure from other etiologies, such as upper or lower airway obstructions, foreign body aspiration and hyperviscosity syndromes [12]. In children with respiratory failure, ECMO is generally used as a rescue therapy and there is a tendency to provide earlier and earlier ECMO support, even in minimally sedated, spontaneously breathing patients [6,12].
In the absence of significant hemodynamic instability, VV-ECMO is usually sufficient to augment gas exchanges, allowing ventilator settings to be progressively weaned and, thus, potentially reducing ventilator-induced lung injury and oxygen toxicity [12]. VV-ECMO has plausible advantages in pediatric patients with good cardiac function, namely preservation of more physiological hemodynamics, better neurological outcome, easier mobilization and a lower complication rate, once it can be established without cannulation of any artery [12,16,18]. The use of ECMO for pediatric asthma has limited retrospective data that suggest excellent survival with relatively shorter time spent on ECMO, but offers little insight concerning potential morbidity or mortality benefit [7,8,11]. Although few evidence exists regarding the ideal mode of ventilation while receiving ECMO, a lung rest approach seems to avoid any further exacerbation of ventilator-induced lung injury and subsequent further inflammation [11,13].
Pediatric patients on ECMO have traditionally been maintained deeply sedated and, sometimes, paralyzed [12]. Excessive pharmacologic sedation and unnecessary paralysis have been increasingly associated with possible circulatory complications, showing also a strong correlation with delirium [2,12]. They have also been considered important risk factors for increased morbidity and mortality in critically ill populations, whichsupports the recent paradigm shift within the PICU community toward less sedation and earlier mobilization [2, 12]. Besides de-escalation of pharmacologic sedation in the absence of noxious pharyngeal stimulation from the end tracheal tube, extubation during ECMO presents a tantalizing goal with several other benefits, including avoidance of ongoing ventilator induced lung and airway trauma, resolution of air leak, facilitation of airway clearance (through spontaneous breathing and cough), mitigation of neuromuscular deconditioning and improving family bond [11,13,17].
Nevertheless, and despite the growing interest, awake ECMO can be associated with severe complications due to the potentially higher risk of cannula dislocation resulting from active movements of the conscious patient [2,6,16]. Although this concern of dislodgement is theoretically higher in children, there are emerging studies demonstrating that awake ECMO can be safely performed in this population [12,16]. Thus, good fixation of the cannula is of utmost importance to allow mobilization and the practice of awake ECMO requires significant team preparation and mindful balance of potential risks andbenefits on an individualized basis [13,16,19]. The authors present the first successful trial of pediatric awake ECMO in their PICU. This report corroborates the actual evidence that, in selected patients, extubation and appropriately decreased sedation is safe and does not itself compromise the effectiveness of extracorporeal life support [13].
Despite the increasing list of successful case reports, careful patient selection remains the most important single factor to be considered by ECMO providers [12,20]. In fact, our patient revealed an exquisite level of collaboration for her age, what decisively contributed not onlyto the success of the treatment, but also to the absence of complications with both the cannulas and the circuit of ECMO. Even though our patient’s recovery was fast, the concept of awake ECMO can be applied over longer periodsand, for now, its benefits are believed to fairly exceed the risks [2,19,21,22]. Further reports and multicenter research are essential to refine the management of children supported on ECMO [2,12,23]. In the near future, it is likely that awake ECMO and early mobilization will become the new standard of care for pediatric patients with respiratory failure [2,13,24]. But for now, it is in our hands not only to accurately predict which patients can be saved by ECMO, but also to minimize as much as possible the inherent morbidity, allowing these children, when appropriate, to be awake, extubated and even ambulatory [12,18].
Acknowledgements: There is nothing worth mentioning in this section.
References
- Erdil T, Lemme F, Konetza A, et al. Extracorporeal membrane oxygenation support in pediatrics. Ann Cardiothorac Surg. 2019; 8(1): 109-115.
- Zhao Z, Li L, Liu Y, et al. Application of Awake Extracorporeal Membrane Oxygenation in Pediatric Acute Fulminant Myocarditis: A Single-Center Experience. J TranslCrit Care Med. 2021; 3: 18.
- Armsby C, Baron EL, Barss VA, et al. Patient education: Extracorporeal membrane oxygenation (ECMO) (The Basics). Available at: https://www.uptodate.com. Accessed April 7, 2022.
- Toh TSW, Ong C, Mok YH, et al. Nutrition in Pediatric Extracorporeal Membrane Oxygenation: A Narrative Review. Front. Nut. 2021; 8: 666464. [DOI: 10.3389/fnut.2021.666464].
- Rajapreyar P, Castaneda L, Thompson NE, et al. Association of Fluid Balance and Survival of Pediatric Patients Treated With Extracorporeal Membrane Oxygenation. Front. Pediatr. 2021; 9: 722477. [DOI: 10.3389/fped.2021.722477].
- Amodeo I, Nardo MD, Raffaeli G, et al. Neonatal Respiratory and Cardiac ECMO in Europe. European Journal of Pediatrics. 2021; 180: 1675-1692. [DOI: 10.1007/s00431-020-03898-9].
- Greenawald L, Strang A, Froehlich C, et al. Status asthmaticus requiring extracorporeal membrane oxygenation associated with rhinovirus infection. Journal of Asthma. 2019. [DOI: 10.1080/02770903.2019.1565826].
- Bizzintino J, Lee W, Laing I, et al. Association between human rhinovirus C and severityof acute asthma in children. EurRespir J. 2011; 37: 1037-1042.
- Cui Y, Zhang Y, Dou J, et al. Venovenousvs. Venoarterial Extracorporeal Membrane Oxygenation in Infection-Associated Severe Pediatric Acute Respiratory Distress Syndrome: A Prospective Multicenter Cohort Study. Front. Pediatr. 2022; 10: 832776. [DOI: 10.3389/fped.2022.832776].
- Maeda K, Ryan K, Conrad CK, et al. An alternative cannulation approach for venovenous extracorporeal membrane oxygenation in children for long-term ambulatory support. J ThoracCardiovascSurg. 2018; 156: e13-4. [DOI: 10.1016/j.jtcvs.2018.03.099].
- Lin JC. Extracorporeal Membrane Oxygenation for Severe Pediatric Respiratory Failure. Respir Care. 2017; 62(6): 732-750.
- Wong JJ, Cheifetz IM, Lee JH. Extracorporeal membrane oxygenation for severe pediatric respiratory failure. J EmergCrit Care Med. 2017; 1: 11. [DOI: 10.21037/jeccm.2017.07.01].
- Costa J, Dirnberger DR, Froehlich CD, et al. Awake Neonatal Extracorporeal Membrane Oxygenation. ASAIO Journal. 2019. [DOI: 10.1097/MAT.0000000000001029].
- Goto T, Suzuki Y, Suzuki Y, et al: The Impact of Extracorporeal Membrane Oxygenation on Survival in Pediatric Patients With Respiratory and Heart Failure: Review of Our Experience. Artificial Organs. 2011; 35(11): 1002-1009. [DOI: 10.1111/j.1525-1594.2011.01374.x].
- Weber G, Schock S, Fox KA, et al. ECMO cannulation in a non-intubated child. Research Square. 2022. [DOI: 10.21203/rs.3.rs-1582120/v1].
- Schmidt F, Sasse M, Boehme M, et al. Concept of “awake venovenous extracorporeal membrane oxygenation” in pediatric patients awaiting lung transplantation. Pediatr Transplantation. 2013; 17: 224-230. [DOI: 10.1111/petr.12001].
- Deng L, Xia Q, Chi C, et al. Awake veno-arterial extracorporeal membrane oxygenation in patients with perioperative period acute heart failure in cardiac surgery. J Thorac Dis. 2020; 12(5): 2179-2187. [DOI: 10.21037/jtd.2020.04.38].
- Valencia E, Nasr VG. Updates in Pediatric Extracorporeal Membrane Oxygenation. Journal of Cardiothoracic and Vascular Anesthesia. 2019; 000: 1-15. [DOI: 10.1053/j.jvca.2019.09.006].
- Schmidt F, Sasse M, Boehne M, et al. Pushing the envelope: “Awake Venoarterial Extracorporeal Membrane Oxygenation” in Pediatric Patients with Acute Cardiac Failure. Pediatr Transplantation. 2013; 17: 224-230. [DOI: 10.1111/petr.12001].
- Robinson S, Peek G. The role of ECMO in neonatal & pediatric patients. Paediatrics and Child Health. 2015. [DOI: 10.1016/j.paed.2015.03.005].
- Turner DA, Rehder KJ, Bonadonna D, et al. Ambulatory ECMO as a Bridge to Lung Transplantation in a Previously Well Pediatric Patient with ARDS. Paediatrics. 2014; 134: e583. [DOI: 10.1542/peds.2013-3435].
- Trento A, Thompson A, Siewers RD, et al. Extracorporeal membrane oxygenation in children. J Thorac Cardiovasc Surg. 1988; 96: 542-7.
- Schmidt F, Jack T, Kaussen T, et al. “Awake Veno-arterial Extracorporeal Membrane Oxygenation” in Pediatric Cardiogenic Shock: A Single-Center Experience. Pediatr Cardiol. 2015; 36: 1647-1656. [DOI: 10.1007/s00246-015-1211-8].
- Fletcher K, Chapman R, Keene S. An overview of medical ECMO for neonates. Seminars in Perinatology. 2018; 42: 68-79. [DOI: 10.1053/j.semperi.2017.12.002].

