Original Article - Volume 2 - Issue 6
Management of children with operable biliary atresia, single center experience
Mohammed Abdel-Hafez Ali1*; Marwa Sabry Rizk1; Salma Abdel Megeed Nagi1; Islam Ismail Ayoub2; Bassam Abdel Hakam Ayoub1
1Department of Pediatric Hepatology, Gastroenterology and Nutrition, National Liver Institute, Menoufia University, 32511 Shebin El-Kom, Menoufia, Egypt.
2Department of Hepatobiliary, Pancreatic and Liver Transplantation Surgery, National Liver Institute, Menoufia University, 32511 Shebin El-Kom, Menoufia, Egypt.
Received Date : Oct 07, 2022
Accepted Date : Nov 17, 2022
Published Date: Dec 10, 2022
Copyright:© Mohammed Abdel-Hafez
Ali 2022
*Corresponding Author : Mohammed Abdel-Hafez Ali, Department of Pediatric Hepatology, Gastroenterology and Nutrition, National Liver Institute, Menoufia University, 32511 Shebin El-Kom, Menoufia, Egypt. Tel: +20 100-2362-768; Fax: +2-048- 226- 8431
Email: abdelhafez64@yahoo.com
DOI: Doi.org/10.55920/2771-019X/1318
Abstract
Introduction: Biliary Atresia (BA) is a devastating pediatric cholangiopathy affecting the bile ducts of the liver. Early diagnosis of BA is important, as the negative impact of late surgery is worse coming.
Aim: We aimed to highlight the characteristics, management strategies and outcome of BA patients managed in our center.
Materials and Methods: This retrospective study was conducted on 100 patients diagnosed with BA had undergone hepatic porto-enterostomy (HPE). The data were collected from patients' records. Data collection comprised patients demographic, the onset of neonatal jaundice, color of urine and stool, associated congenital anomalies, biochemical, imaging and histopathological findings, management, the surgical type of BA, follow-up data and any recorded complications.
Results: The study included 100 infants with BA had undergone surgical repair, 50% were males, 50% were females, and their mean age at surgery was 78.03±15.71 days. All patients initially presented with clay stool and jaundice; 29% developed jaundice at birth and the remaining developed jaundice their after. The pre-operative laboratory findings showed elevation in liver functions and alpha fetoprotein and ferritin levels which gradually decreases after surgery. The ultrasound assessment revealed that; 74% of cases had hepatomegaly, 48% had splenomegaly. 48% had atreticgallbladder and 33% were of the non-contractile type. Hepatic subcapsular flow was present in 25% of cases and the Triangular cord (TC) sign was Positive in 29%. The histopatological findings revealed that; more than half of the cases had advanced stages of fibrosis and mild activity, ductal proliferation was present in 99% of cases and bile plugs were shown in 98% of patients. On following up the patients 6 months post-operative 29% had achieved successful outcome while 71% were failed. 78 cases (78%) had survived and 22 cases (22%) died.
Conclusion: BA is a devastating disease affecting newborns, there is a progress in BA diagnosis however there is a dire need for specific therapy development to achieve better outcome.
Keywords: Atreticgallbladder, Biliary atresia,Histopatological findings, Hepatic porto-enterostomy.
Introduction
Biliary Atresia (BA) is a form of cholangiopathy that affects both the extra- and intrahepatic bile ducts. The incidence is particularly high in Asia (100-500 per 100,000 live births in Taiwan and Japan [1] as compared to Europe (5-25 per 100,000 live births) [2]. The etiopathogenesis of BA is multifactorial and multiple pathomechanisms have been proposed. Experimental and clinical studies have suggested that viral infection initiates biliary epithelium destruction and release of antigens that trigger a T helper-1 (Th1) immune response, which leads bile duct injury, progressing to inflammation and obstructive scarring of the biliary tree. It has also been postulated that BA is caused by a defect in the normal remodeling process. Genetic predisposition has also been proposed as a factor for the development of BA [3].
The diagnosis of BA is still terrifying at the 3rd decade of the 21st century, parents and physicians face a challenge with a jaundiced baby, who may or may not have a surgically correctable cholangiopathy. Systematic approaches have been evaluated, despite that the etiology still ambiguous. The primary interest with this disease is to identify the etiology and change the treatment from symptomatic to curative [4]. The current treatment options are limited to a surgical procedure called hepatic porto-enterostomy (HPE), but HPE fails to improve the condition in nearly 50% of the patients and leaves the intrahepatic cholangiopathy unresolved. Moreover, BA leads to liver fibrosis, portal hypertension and hepatic failure in a lot of cases. Ultimately, liver transplantation is often needed, requiring life-long immunosuppression, which affects the quality of life for BA patients [5]. We aimed to highlight the characteristics, management strategies and outcome of BA patients managed in our center.
Materials/Methods
This was a retrospective study conducted on 100 patients diagnosed with BA at National liver institute, Menoufia University, Egypt between October, 2015 and October, 2020. The data were collected from patients' records in the Pediatric Hepatology and Hepatobiliary, Pancreatic and Liver transplantation surgery departments following approval of the Research Committee at National liver institute, Menoufia University, Egypt. Data collection comprised patients demographic, the onset of neonatal jaundice (since or after birth), color of urine and stool, associated congenital anomalies, biochemical, imaging and histopathological findings, management strategy, the surgical types of BA, follow-up data and any recorded complications.
Laboratory data included liver function tests (LFTs) namely; Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Gamma-Glutamyl Transferase (GGT), Alkaline Phosphatase (ALKP), total and direct bilirubin, and serum albumin. Hematological data included total leukocyte count, hemoglobin, platelet count, prothrombin time, and international normalized ratio (INR).The previous testswere evaluated at diagnosis and at 1, 3 and 6 months after the corrective surgery. TORCH screening results on admission were collected.
Ultrasonographic (US) Imaging findings of a normal or abnormal gallbladder, hepatomegaly, liver echotexture, common bile duct visualization, splenomegaly, signs of portal hypertension, Hepatic subcapsular flow and triangular cord (TC) sign, were recorded. The results of the wedge Liver biopsy were reported. Histopathologic findings of bile plugs, intracanalicular cholestasis, bile ductular proliferation (with and without portal fibrosis), and the absence of giant cell transformation were considered suggestive for BA diagnosis in the appropriate clinical context. The BA diagnosis was confirmed when the intra-operative cholangiography showed the typical findings of complete obliteration of the common bile duct or the extrahepatic bile duct.
All patients included in the study had received the same postoperative medical and nutritional management during the study period and included: prophylactic antibiotics in the form of intravenous 3rd generation cephalosporin plusmetronidazole till the 6th postoperative day then oral cotrimoxazole for 6 months, ursodeoxycholic acid (10–15 mg/kg divided into 3 doses); a choleretic drug that was given with the beginning of oral intake for 3months and lipid-soluble vitamins were given for 3months. Successful HPE was considered when establishment of bile flow had achieved 6 months after surgery as evidenced by total bilirubin concentration ≤2 mg/dl [6].
Statistical analysis
The data were analyzed using the Statistical Package for the Social Sciences (SPSS) software, Version 20 (IBM Corp., Armonk, NY, USA). The descriptive analysis incorporated numbers and percentages for categorical data and minimum, maximum, means with standard deviation (SD) for continuous variables. The chi-square test was used to compare categorical variables. The independent sample t-test or Mann whitteny test were used to compare 2 sets of quantitative data as appropriate. Kruskal-Wallis test was used to compare more than 2 sets of quantitative data, non-parametrically distributed. A paired samples t-test and Wilcox on signed rank test were used to assess the follow up of the laboratory parameters before and after the Kasai procedure, as appropriate. Survival was assessed using the Kaplan–Meier method. P < 0.05 was considered statistically significant.
Results
The study included 100 infants with BA had undergone surgical repair, 50% were males, 50% were females, their mean age at surgery was 78.03±15.71 days, ranging from 31-141 days. The mean of the pre-operative weight was 4.67±0.6 Kg, The mean of the pre-operative height was 57.6±3.4 cm. All patients initially presented with clay stool and jaundice; 29% developed by jaundice at birth and the remaining developed jaundice their after. One case had associated intracranial hemorrhage. Associated cardiovascular anomalies were present in 3 cases, another 3 cases had polysplenia, 2 cases had pre duodenal portal vein and 1 case had malrotated colon.
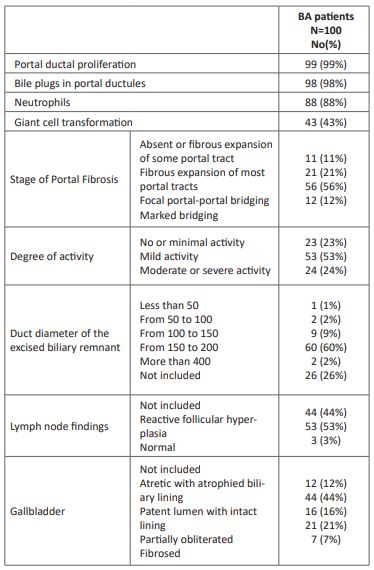
The pre-operative laboratory findings showed elevation in bilirubin, AST, ALT, ALKP, GGT and alpha fetoprotein (AFP) and ferritin levels (Table 1). Positive antibodies in TOURCH screening were; RubellaIgM (3.1%), RubellaIgG (81.9%), ToxoplasmaIgM (1.2%), ToxoplasmaIgG (39.8%), herps simplex (HSV)1IgG(57.6%), HSV2IgG (58.6%), cytomegalovirus (CMV) IgM (35.6%), CMV IgG (52.7%). Polymerase chain reaction for CMV was positive in15.9% of patients.
Table 1: Pre-operative laboratory parameters of the BA patients.
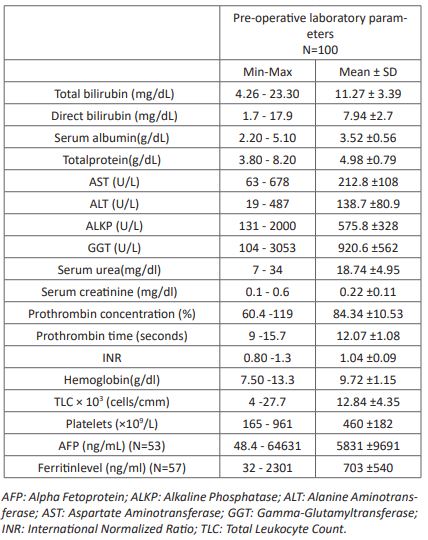
Table 2: The histopatological findings of the wedge liver biopsy and the excised tissues.
The ultrasound assessment revealed that; 74% of cases had Hepatomegaly, 48% had Splenomegaly, 1 case had multiple splenules, and ascites was present in 2 cases. 48% had atretic gallbladder and 33% were of the non-contractile type. Cystic changes in an obliterated biliary tract was present in 4% of cases and the remaining cases showed normal gallbladder contractility. Hepatic subcapsular flow was present in 25% of cases and the triangular cord (TC) sign was positive in 29%. The histopatological findings revealed that; more than half of the cases had advanced stages of fibrosis and mild activity, ductal proliferation was present in 99% of cases and bile plugs were shown in 98% of patients (Table 2). Tubal duodenal aspirate showed no bile in 97% of case and the remaining 3 cases had only tinge of bile. Their BA score was 23.6±3.5 (ranging from 11.1 to 33.12). Regarding the surgical Type of BA; 73% of the cases were type 3 BA, 16% were type 2 BA, 6% were type 1, 4% were cystic BA and 1% was BA with choledocal cyst. 79% of the cases had undergone kasaiporto-enterostomy, 16% chelecysto-portostomy and 5% hepatico-jejunostomy.
Postoperatively; LFTs showed gradual decrease (P <0.0001) (Table 3). Manifestations of hepatic decompensation appeared in 26% of cases. burst abdomen occurred in one case and recurrent cholangitis occurred in 54% of patients. 25% of patient had developed growth failure post-operative. On following up the patients 6 months post-operative 29% had achieved successful HPE while 71% were failed. 78 cases (78%) had survived and 22 cases (22%) died (Figure 1). 16 cases died of liver failure,3 cases died with pneumonia 2 cases died of septicemia and 1 case died due to intestinal obstruction. The age at surgery, gender distribution and anthropometric measurements were comparable between patients with successful and failed outcome (P> 0.05) (Table 4). While significant growth failure occurred in patients with failed outcome (36%) than successful outcome (0%) (P <0.0001).
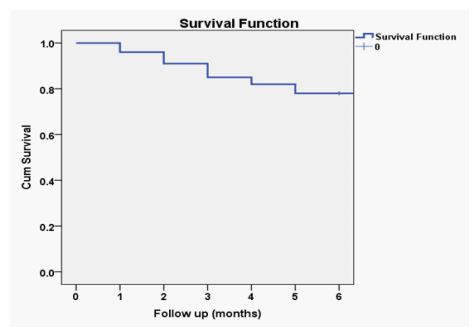
Figure 1: The 6th months´ patient survival after the corrective surgery.
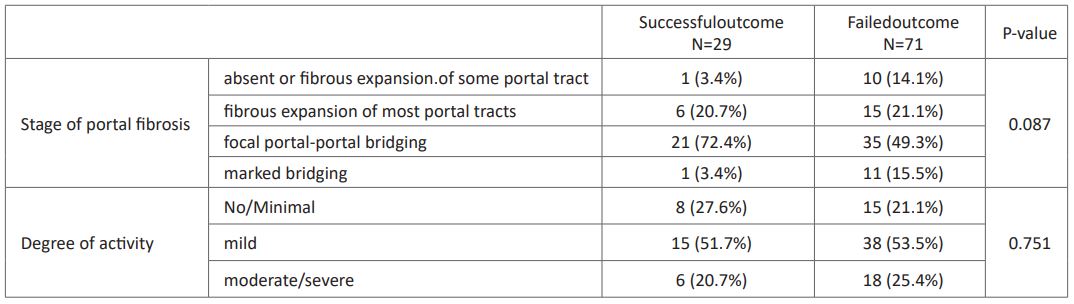
On comparing the pre-operative laboratory findings between patients with successful and failed outcome; there was no statistical significant difference between patients with successful and failed outcome as regard the LFTs nor the CBC parameters (P> 0.05) (Table 4). Presence of atretic gallbladder was significantly higher in patient with failed outcome than those with successful outcome in both ultrasound and histopathology (P<0.05), while the remaining ultrasound and histopathological findings didn't show higher prevalence in certain outcome even the degree of liver fibrosis and activity were not predictable for outcome of surgery (P> 0.05) (Table 5). BA score did not differ significantly between failed outcome (24.1±3.1) and successful outcome (22.5±4.1) (P> 0.05).
Table 3: The follow up of the liver function test of the BA patients; pre-operative, 1 month, 3 months and 6 months post-operative.
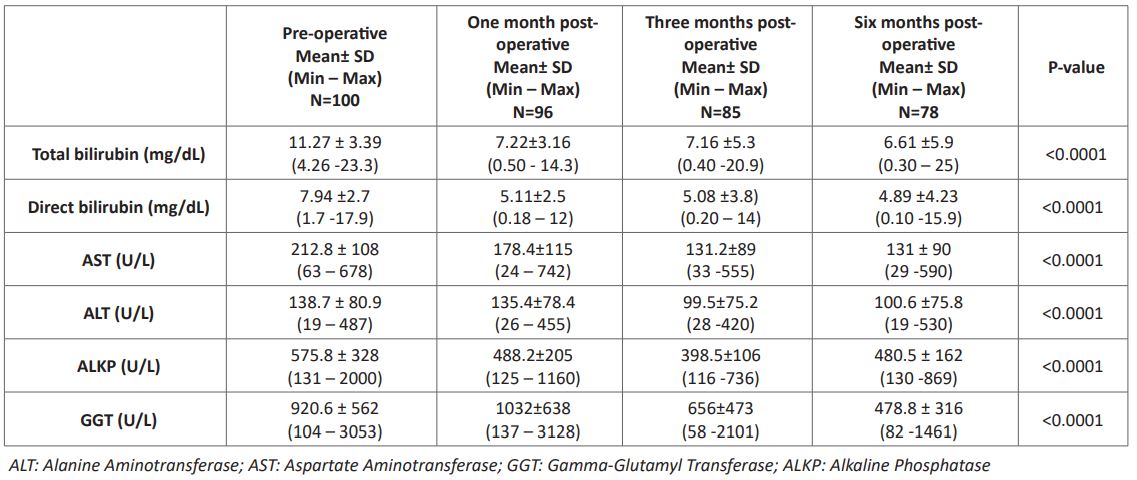
Table 4: Comparison of theage at surgery,anthropometric measurements and pre-operative laboratory findings between patients with successful and failed outcome.
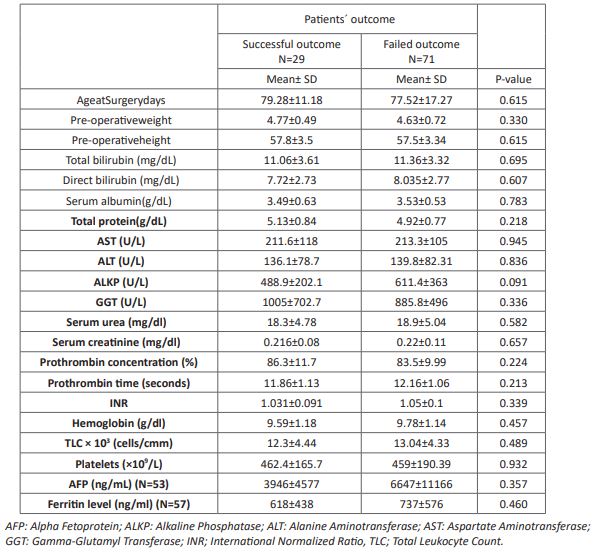
Table 5: stage liver fibrosis and degree of activityin patients with successful and failed outcome.
Discussion
Biliary Atresia is a devastating pediatric cholangiopathy affecting the bile ducts of the liver which can present with different phenotypes [7]. Our BA patients showed 3 clinical phenotypes; nonsyndromic phenotype (87%), syndromic phenotype (9%) and cystic variant (4%) of cases. The main congenital malformations associated with the syndromic phenotype waspolysplenia, cardiovascular anomalies, pre-duodenal portal vein and malrotated colon. Clinical studies reported that; the non-syndromic or isolated form of BA is the most common form and accounts for 70–80% of cases [8]. The syndromic or congenital form is associated with extrahepatic developmental anomalies as asplenia, polysplenia, double spleen, cardiovascular defects, situsinversus, intestinal malrotation, small-intestinal atresia, pancreaticobiliary maljunction and various positional abnormalities of the portal vein and hepatic artery and is referred to as BASM syndrome, which accounts for 10% of cases [9].Cystic BAis relatively uncommon but notable variant of BA and accounts for approximately 5%-10% of BA cases [10].
A number of viruses, including CMV, epstein-Barr virus and human papillomavirus have been linked to BA [11]. Presence of positive IgM to CMV was a significant association in nearly one third of our cases, which alarms our minds for the possible role of CMV in the pathogenesis of BA. Liver T cell responses to CMV were identified in a majority of BA patients at diagnosis, suggesting perinatal CMV infection as an initiator of bile duct damage [12].
The importance of early diagnosis of BA is well recognized [13], as the negative impact of late surgery has been demonstrated in previous studies [14]. Signs and symptoms related to poor bile flow asclay stool and jaundice were the main signs observed by the parents of our patients and led them to seek medical advice; both are present in 100% of the patients. The examination of bile-stained stool in infants with suspected BA is a non-invasive method for suspicion of BA. Some countries adopted the policy of universal screening of infant stool colour and reported promising results [13].
Laboratory analyses revealed direct hyperbilirubinemia, variable levels of transaminases, GGT, alkaline phosphatase, AFP and ferritin levels, which overlap with other causes of neonatal cholestasis, so more work up for diagnosis are warranting. Work-up for suspicion of BA is extensive. Once blood and stool samples are obtained, abdominal ultrasound is used to confirm diagnosis and exclude other potential causes as ultrasound assessment plays an important role in the diagnosis of BA. In the current study; presence of the atretic or the non-contractile type gallbladder (81% of cases) is considered a key in diagnosis of BA. Presence of hepatic subcapsular flow and the TC sign in a cholestatic infant added more evidence for the diagnosis of BA.
Ultrasonography, which is a radiation-free, noninvasive and real time imaging modality, has been recommended as the preferred imaging tool for the initial detection of BA [15]. A Meta-Analysis assessing the role of ultrasound in the diagnosis of BA, reported that; the TC sign and gallbladder abnormalities are the two most accurate and widely accepted ultrasound characteristics for diagnosing or excluding BA. Other ultrasound characteristics are less valuable for diagnosis or exclusion of BA [16]. Tubal duodenal aspirate is a routine investigation in our center for any infant presenting with jaundice and clay stool, or had high incidence of suspicion for BA. Nearly, the yellow biliary duodenal fluid wasn't observed in all of the patients. Absence of bile in the aspirate was in favor of diagnosis of BA.
The main histopathological findings for BA diagnosis are present in the portal tracts. Advanced stages of fibrosis, ductal proliferation and bile plugs were the predominant findings in our patients. It is worth noting, that the earliest histologic changes seen in BA are nonspecific, and a liver biopsy done in the very early stages of the disease process may lead to false negative results [17]. Russo et al., on assessing the histopathologic features of liver biopsies that distinguish BA from other causes of infantile cholestasis found that portal tracts show expansion and demonstrate edematous fibroplasia associated with bile ductular proliferation, that consist of anastomosing ductules present at the periphery of portal tracts. This finding represents the most consistent indicator of the presence of a biliary obstructive process [18].
Patients with high BA score which consists of collection of the previous diagnostic methods were transferred to the surgical department for confirmation of diagnosis by intraoperative cholangiography; the gold standard for BA diagnosis and doing the corrective surgery for cases with proved diagnosis. Type III BA was the comments type of the studied group followed by type II BA. Most of the cases had undergone Kasai porto-enterostomy while small percentage managed with chelecysto-portostomy and hepatico-jejunostomy.
The porto-enterostomy indicated by the Japanese Morio Kasai, represents a breakthrough for the outcome of patients affected with BA, however the risk of cholangitis and other several factors may jeopardize the outcome [19]. Hepatic decompensation, recurrent cholangitis and growth failure were the main encountered complications during the period of the study. The entire mechanism for the occurence of cholangitis is unknown. But, the formation of a Roux-en-Y loop with restoration of bilio-intestinal flow lead to colonization of the loop with bacteria that predispose to ascending cholangitis. Cholangitis is reported in up to 50% of cases [20].
Presence of atretic gallbladder was the only factor predicting bad outcome in the current study, while the other studied parameters were comparable between patients with successful and failed outcomes. After the first 6 months post-operative, Kasai operation was successful on nearly one third of cases and the mortality rate reached 22%. Complications of liver failure were the main cause of mortality followed by infections. Khayat et al., assessed the outcomes of late HPE in 23 BA patients, Their median age at the procedure was 77 ± 34 days, the Kasai procedure was successful in six patients (27%),Thirteen (56%) patients survived with their native livers, 3 (13%) received a transplant, and 6 died (26%) while awaiting a transplant [21].
Conclusion
BA is a devastating disease affecting newborns, there is a progress in BA diagnosis however there is a dire need for specific therapy development to achieve better outcome.
Conflict of interest: Non Declared.
Financial support: Non-Fundable Study.
References
- Tiao MM, Tsai SS, Kuo HW, Chen CL, Yang CY. Epidemiological features of biliary atresia in Taiwan, a national study 1996-2003. Journal of Gastroenterology and Hepatology (Australia). 2008; 23: 62-66.
- Davenport M, De Ville, de Goyet J, Stringer M, Mieli-Vergani G, Kelly D, McClean P. Seamless management of biliary atresia in England and Wales (1999–2002) The Lancet. 2004; 363: 1354-1357.
- Vij M, Rela M. Biliary atresia: pathology, etiology and pathogenesis. Future Sci OA. 2020; 6(5): FSO466.
- Sergi CM, Gilmour S. Biliary Atresia: A Complex Hepatobiliary Disease with Variable Gene Involvement, Diagnostic Procedures, and Prognosis. Diagnostics (Basel). 2022; 12(2): 330.
- Lendahl U, Lui VCH, Chung PHY, Tam PKH. Biliary Atresia - emerging diagnostic and therapy opportunities. EBioMedicine. 2021; 74: 103689.
- Hadzic N. Medical management of the ‘failing’ Kasai portoenterostomy. S Afr Med J. 2012; 102: 868-871.
- Asai A, Miethke A, Bezerra JA. Pathogenesis of biliary atresia: defining biology to understand clinical phenotypes. Nat Rev GastroenterolHepatol. 2015; 12: 342-352.
- Lakshminarayanan B, Davenport M. Biliary atresia: a comprehensive review. J. Autoimmun. 2016; 73: 1-9.
- Asai A, Miethke A, Bezerra JA. Pathogenesis of biliary atresia: defining biology to understand clinical phenotypes. Nat. Rev. Gastroenterol. Hepatol. 2015; 12(6): 342-352.
- Caponcelli E, Knisely AS, Davenport M. Cystic biliary atresia: an etiologic and prognostic subgroup. J Pediatr Surg. 2008; 43: 1619-1624.
- Averbukh LD, Wu GY. Evidence for Viral Induction of Biliary Atresia: A Review. J ClinTranslHepatol. 2018; 6(4): 410-419.
- Brindley SM, Lanham AM, Karrer FM, Tucker RM, Fontenot AP, Mack CL. Cytomegalovirus-specific T-cell reactivity in biliary atresia at the time of diagnosis is associated with deficits in regulatory T cells. Hepatology. 2012; 55(4): 1130-1138.
- Williams R, Alessi C, Alexander G, Allison M, Aspinall R, Batterham RL, et al. New dimensions for hospital services and early detection of disease: a Review from the Lancet Commission into liver disease in the UK. The Lancet. 2021; 397: 1770-1780.
- Nio M, Wada M, Sasaki H, Tanaka H. Effects of age at Kasai portoenterostomy on the surgical outcome: a review of the literature. Surgery Today. 2015; 45: 813-818.
- Hartley JL, Davenport M, A Kelly D. Biliary atresia. Lancet. 2009; 374: 1704-1713.
- Zhou L, Shan Q, Tian W, Wang Z, Liang J, Xie X. Ultrasound for the Diagnosis of Biliary Atresia: A Meta-Analysis. AJR Am J Roentgenol. 2016; 206(5): W73-82.
- Azar G, Beneck D, Lane B, Markowitz J, Daum F, Kahn E. Atypical morphologic presentation of biliary atresia and value of serial liver biopsies. J PediatrGastroenterolNutr. 2002; 34: 212-215.
- Russo P, Magee JC, Anders RA, Bove KE, Chung C, et al. Childhood Liver Disease Research Network (ChiLDReN). Key Histopathologic Features of Liver Biopsies That Distinguish Biliary Atresia From Other Causes of Infantile Cholestasis and Their Correlation With Outcome: A Multicenter Study. Am J SurgPathol. 2016; 40(12): 1601-1615.
- Garcia AV, Cowles RA, Kato T, Hardy MA. Morio Kasai: A remarkable impact beyond the Kasai procedure. J. Pediatr. Surg. 2012; 47: 1023-1027.
- Davenport M, Caponcelli E, Livesey E, Hadzic N, Howard E. Surgical outcome in biliary atresia: etiology affects the influence of age at surgery. Ann Surg. 2008; 247(4): 694-8.
- Khayat A, AlamriAM, Saadah OI. Outcomes of late Kasai portoenterostomy in biliary atresia: a single-center experience. J Int Med Res. 2021; 49(5): 3000605211012596.

