Case Report - Volume 2 - Issue 6
Feasibility of one-lung ventilation using two ventilators in a bleomycin treated patient under normoxic conditions: A case report
Pawlik MT1*; Meyringer H1; Heiligensetzer A2; Niessen C3; Blecha S4; Seyfried T5
1Department of Anesthesiology and Intensive Care Medicine, St. Joseph Hospital, Academic Hospital of the University of Regensburg, Germany.
2Department of Visceral and Thoracic Surgery, St. Joseph Hospital, Academic Hospital of the University of Regensburg, Germany.
3Institute of Diagnostic Radiology, St. Joseph Hospital, Academic Hospital of the University of Regensburg, Germany.
4Department of Anesthesiology and Intensive Care Medicine, University Hospital, Regensburg, Germany.
5Department of Anesthesiology, Ernst-von-Bergmann Hospital, Potsdam, Germany.
Received Date : Oct 13, 2022
Accepted Date : Nov 22, 2022
Published Date: Dec 15, 2022
Copyright:© Michael T Pawlik 2022
*Corresponding Author : Michael T Pawlik, Department of Anesthesiology and Intensive Care Medicine, St. Joseph Hospital, Academic Hospital of the University of Regensburg, Germany. Tel: +49-941-782-3610; Fax: 0049-941-782-3615
Email: anesthesiology@csj.de
DOI: Doi.org/10.55920/2771-019X/1322
Abstract
Background: Bleomycin is a chemotherapeutic agent that has been applied successfully for decades in the treatment of testicular tumors. Case reports and studies in animal models suggest that bleomycin increases the lifelong toxicity of lungs to increased oxygen concentrations, which may trigger ARDS. Therefore, the general recommendation is to keep oxygen levels as low as possible in the context of medical procedures, which opposes the necessity of high oxygen application during one-lung ventilation (OLV).
Case presentation: A 27-year-old male was diagnosed for testicular cancer and treated with five cycles of bleomycin. One year later he underwent open thorax surgery for resection of a large lymphoma in the left thoracic cavity. One-lung ventilation was used, in which one lung is ventilated while the other is allowed to collapse, providing optimal conditions for surgery. Total intravenous anesthesia was perforned, FiO2 was set to 0.23 and a second ventilator was used for high-frequency low-tidal ventilation to result in a ‘collapsed’ left lung. Oxygen saturation remained above 90% and surgery was performed without complications. Recovery of the patient was unremarkable from his extubation to discharge from hospital.
Conclusions: In a patient after bleomycin chemotherapy, an FiO2 of 0.23 and supportive, selective low-tidal high-frequency ventilation of the collapsed lung with a second ventilator may be a viable anesthetic procedure and a trial is justified. Complications during the procedure should be discussed in advance with the team, and the anesthesiologist must be prepared for a sudden transition to regular ventilation of both lungs.
Keywords: Bleomycin lung injury; high frequency ventilation; hypoxic pulmonary vasoconstriction; one lung ventilation.
Abbreviations: ARDS: Adult Respiratory Distress Syndrome; BLI: Bleomycin Lung Injury; CPAP: Continuous Positive Airway Pressure; DLV: Double Lung Ventilation; FiO2: Fraction Of Inspired Oxygen; HPV: Hypoxic Pulmonary Vasoconstriction; MAC: Minimal Alveolar Concentration; OLV: One-Lung Ventilation; TLC: Total Lung Capacity.
Background
Bleomycin is an effective chemotherapeutic from a group of antibiotics that was first synthesized in 1966 and is used successfully in the therapy of Hodgkin's lymphoma and testicular tumors [1]. The most important side effect, which may be fatal for the patient, is damage to the lungs; this can manifest itself both in fibrotic changes and in unspecific inflammatory processes - up to and including acute lung failure (ARDS) [2]. These effects are summarized under the term bleomycin lung injury (BLI). The incidence is reported in the literature as between 0% and 46%, and the mortality rate among those affected varies from 2% to 10%, but increases up to 33% under mechanical ventilation [2]. In addition, a number of animal studies suggests that previous exposure to bleomycin leads to increased oxygen toxicity [3]. This observation is supported by pathophysiological considerations, since the mechanism of action of bleomycin consists in the release of oxygen radicals with subsequent DNA damage in the affected cells [4, 5].
For patients undergoing general anesthesia for surgery after bleomycin therapy, this has important implications for the perioperative procedure, since the rate of postoperative pulmonary complications appears to be significantly increased. Strategies to avoid excessive fluid intake and ventilation regimes with low FiO2 were established in the last few decades. A publication by Goldiner et al. suggested the advantage of such an approach as early as the late 1970s [6]. Further studies on perioperative oxygen restriction did not confirm their superiority. In a prospective study with 361 patients, Aakre et al. showed that the incidence of postoperative ARDS after bleomycin therapy with the ventilation strategies commonly used today is lower than previously assumed, but that certain subgroups have a significantly increased risk [7]. In this context, anesthesia for thoracic surgery with one-lung ventilation poses a particular challenge and has not been previously published. On the one hand, optimal surgical conditions with isolation of one lung must be provided, but on the other hand, adequate maintenance of oxygenation must be ensured. If hypoxemia is imminent, countermeasures include increasing the FiO2 of the ventilated lung and selective O2 insufflation of the collapsed lung, with CPAP if necessary [8]. The resulting increased oxygen partial pressure instantly improves oxygenation as well as the effectiveness of HPV, but may increase the risk of postoperative complications after bleomycin therapy.
Case presentation
A 27-year-old patient who underwent orchiectomy in 2018 for mixed testicular cancer received six cycles of systemic chemotherapy with bleomycin, etoposide, and cisplatin (PEB regimen) three years later for multiple para-aortic, mediastinal, and pulmonary metastases. Transperitoneal para-aortic and para-esophageal lymphadenectomy was performed three months after chemotherapy. The para-aortic lymph node conglomerate in the area of the spine, a lung metastasis and a thoracic wall metastasis could not be resected at the initial operation, so in 2019 it was decided to carry out a second operation with a left-sided thoracotomy under one-lung ventilation anesthesia.
Preoperative measurements of lung function revealed a TLC of 6.1 L (84% of normal), FEV I 2.69 L (60% of normal), VC 3.12 L (56% of normal), FEV1/VC (Tiffeneau-Pinelli index) 86.30% (105% of target) and a residual volume of 2.99 L (174% of target). Lung diffusion capacity was not examined. The static and dynamic expiratory volumes were worse than those three months previously. A slight restrictive ventilation disorder was found with a decrease in the TLC from 8.46 L to 6.11 L (84%), and the VC fell from 5.58 L (100%) to 3.12 L (56%). Our preoperative diagnosis was bleomycin-induced pulmonary fibrosis. Preoperative oxygen saturation was 96%. The operation was planned under general anesthesia in the form of a total intravenous anesthesia (TIVA) with one-lung ventilation. In an uncomplicated airway known to have been pre-anesthetized, anesthesia was initiated without pre-oxygenation but with morphine, remifentanil, propofol and atracurium, and the anesthesia was maintained with propofol and remifentanil. The patient refused a thoracic peridural catheter.
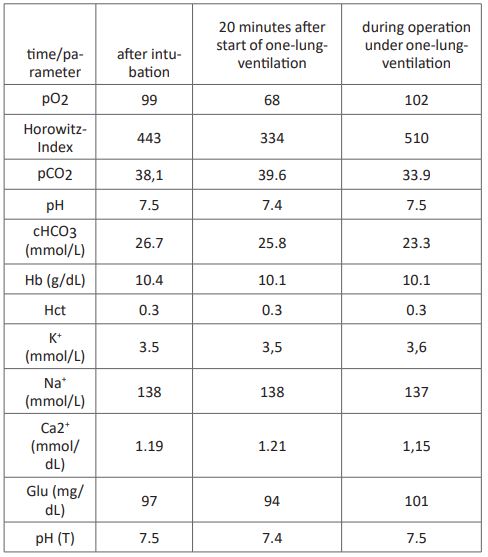
Intubation was performed with a 39 CH left-sided Roberts haw double-lumen tube. After easy positioning, bronchoscopic control showed when the tube was in the correct position. Because of pretreatment with bleomycin therapy and preoperative slight lung restriction, anesthesia was carried out with an average oxygen concentration of 23% throughout the entire period of anesthesia (Atlan® A-350 XL, Dräger, Germany). Standard monitoring was supplemented with an arterial cannula in the left radial artery, and a second peripheral infusion needle was placed at the right forearm. The patient was kept normothermic (≥ 36.5 C) with a convective heat supply (Bair-Hugger®, 3M, Germany). The first arterial blood sample under bi-lung ventilation showed a Horowitz index of 471 with an FiO2 of 0.21 (see Table). To achieve the best possible conditions for the surgeon, we used a second ventilator (Hamilton®-MR1, Hamilton Medical, Germany) and an FiO2 of 0.23 to ventilate the left lung in BIPAP mode with pressure limits 9 and 5 below an FiO2 of 0.22 and a frequency of 80/min (see Table). The resulting respiratory tidal volumes averaged about 80 ml. Lung ventilation showed a pO2 of 70 mmHg (corresponding to a Horowitz index of 319) and an oxygen saturation of 88-90%. After 25 minutes of one-lung ventilation the pO2 was up to 107 mm Hg (corresponding to a Horowitz index of 465). An impending intraoperative hypoxemia was prevented with the described isolated ventilation of the collapsed lung, using minimal breath strokes and a high respiratory rate with 23% inspiratory oxygen partially avoided. Entire resection of the lymph node cluster attached to the vertebral column lasted 80 minutes with a one-lung ventilation time of 30 minutes. The patient was extubated without problems after surgery was complete and admitted to our intensive care unit for 24 hours. He was transferred to the ward on the following morning and discharged from hospital after seven days.
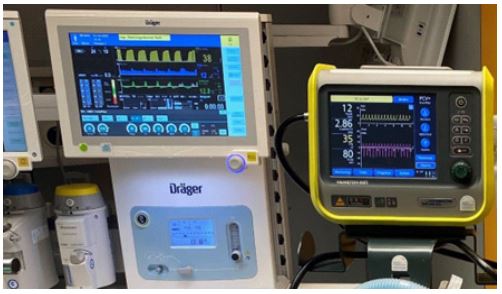
Figure 1: Ventilation devices in the operating theater.
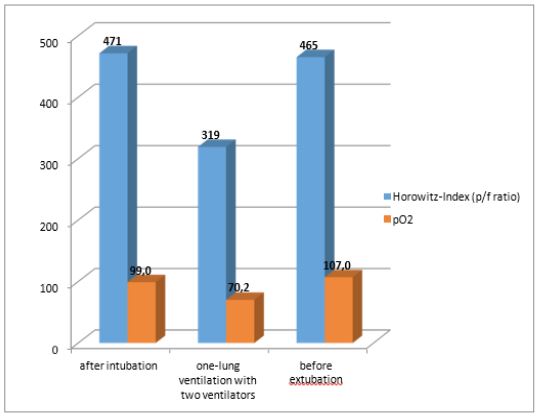
Figure 2: Horowitz Index at different time points A – After intubation FiO2 0,21
B – Blood gas samples 15 minutes after start of one-lung-ventilation
C - During surgery, ventilating the left lung for surgery with FiO2 0.25, BiPAP 12/5, frequency 80/min, AMF 2.86 l/min.
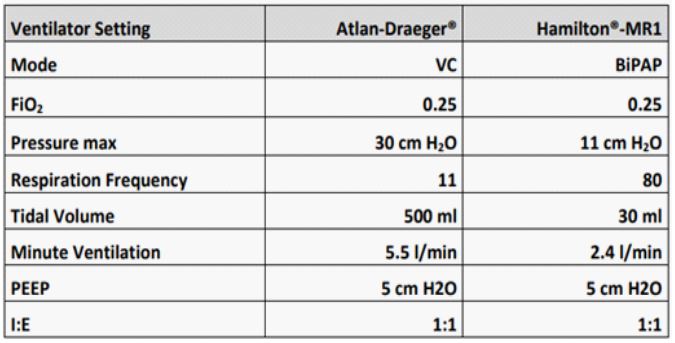
Figure 3: ventilators´setting.
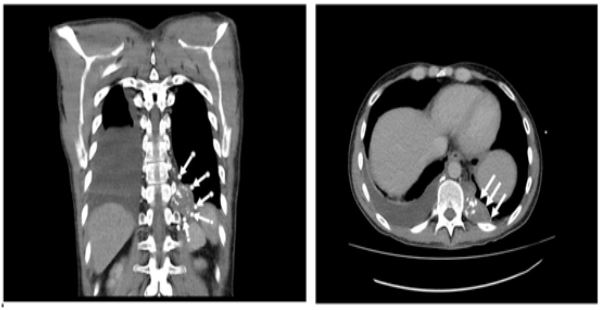
Figure 4: MRI scan: lymphoma of left thoracic cavity (white arrows).
Table 1
Discussion and conclusions
Patients undergoing chemotherapy with bleomycin retain an increased sensitivity of the lung to oxygen damage with increased inspiratory oxygen application for the rest of their lives. In the event of surgery that may be required, the anesthesiologist needs to consider two important issues. Bleomycin-treated lungs may suffer from hypoxemia, which permanently limits quality of life [9]. Furthermore, the use of elevated oxygen levels may be hazardous to patients if there are emergencies or anesthesia during surgery. In this context, oxygen toxicity is a function of dosage and time, i.e. oxygen concentrations resulting in an FiO2 of ≤ 0.25 should be aimed for in order to prevent postoperative development of ARDS [10].
Although recent retrospective data indicate that the risk with about 2% appears to be much lower than previously thought, it is important to avoid elevated oxygen concentrations during anesthesia [7]. Once pulmonary intervention is necessary, one-lung ventilation is desirable to provide the surgeon with the optimal operating conditions created by an immobilized lung [11]. The usual practice is ventilation of the relevant lung with an FiO2 of 0.5 to 0.9 in order to avoid hypoxemia and prevent hypoxic pulmonary vasoconstriction in the ventilated lung [8,12]. We were able to perform one-lung ventilation in a bleomycin-treated patient with an FiO2 of 0.23, achieving oxygenation almost as good as that under spontaneous breathing by using a second ventilator with modified “high-frequency jet ventilation". Maintenance of HPV is the main goal of single-lung ventilation and may be compromised by several factors [13]. These include the use of vasodilator agents such as antihypertensives and volatile anesthetics at concentrations greater than 1.0 MAC. Application of high concentrations of oxygen (> 0.5) to the ventilated lung establishes normoxia and prevents HPV in the ventilated lung due to inadequately ventilated alveolar regions.
One-lung ventilation is commonly performed for severe unilateral thoracic trauma or other complex diseases and has been published in the literature [14,15]. The use of high- frequency ventilation instead of double lumen tubes has been described as feasible during minimally invasive coronary bypass graft surgery [16]. Application of CPAP with 50% oxygen during one-lung ventilation was shown to ensure better oxygenation at a lower pulmonary shunt than high FiO2 without CPAP [17]. However, this approach should not be first choice for bleomycin treated patients. Immobilization of the operated lung was shown to be feasible with the use of a standard PEEP level of 5 cm H2O and a low tidal volume of 80 ml with normoxic oxygen concentrations. Whereas real one-lung ventilation results in an approximate halving of the Horowitz index with HPV [18,19], we were able to produce a sufficient Horowitz index when "functional" immobilization of the lung to be operated was established. Based on the one- lung ventilation and successful normal oxygenation, we consider an attempt to be justified with one-lung ventilation under normoxic oxygen concentrations. The risk to the patient of impending hypoxemia must be reduced by immediate termination of one-lung anesthesia.
Declarations Funding: None.
Competing interests: The authors declare that they have no competing interests.
Acknowledgements: We thank Professor emerit. David Tracey (University of NSW, Australia), for proofreading the article. We also thank the patient for permission to publish his case.
Authors’ contributions: Patient management, data collection: HM, AH, CN Data collection and manuscript drafting: MTP, SB, TS
Manuscript revision: RMJD. All authors approved the final manuscript.
Availability of data and material: All datasets are available from the corresponding author.
Ethical approval and consent to participate: Not applicable.
Consent for publication: The patient was informed about the case report, why the case was special and the authors’ interest in publishing his case. The patient willingly gave informed consent to the use of every image needed for this case report. The authors respect the patient’s anonymity.
Our patient gave written consent for publication of his clinical information and scan image.
References
- Umezawa H, et al. New antibiotics, bleomycin A and B. J Antibiot (Tokyo). 1966; 19(5): 200-9.
- P BBE. Bleomycin Lung Injury: A Case Report and Review of the Literature. Journal of Anesthesia & Intensive Care Medicine. 2017; 3.
- Hay JG, et al. Development of acute lung injury after the combination of intravenous bleomycin and exposure to hyperoxia in rats. Thorax. 1987; 42(5): 374-82.
- Nici L, et al. Modulation of bleomycin-induced pulmonary toxicity in the hamster by the antioxidant amifostine. Cancer. 1998; 83(9): 2008-14.
- Suryadevara V, et al. Role of phospholipase D in bleomycin-induced mitochondrial reactive oxygen species generation, mitochondrial DNA damage, and pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2019; 317(2): L175-L187.
- Goldiner PL and O Schweizer. The hazards of anesthesia and surgery in bleomycin-treated patients. Semin Oncol. 1979; 6(1): 121-4.
- Aakre BM, et al. Postoperative acute respiratory distress syndrome in patients with previous exposure to bleomycin. Mayo Clin Proc. 2014; 89(2): 181-9.
- Senturk M, P Slinger and E Cohen. Intraoperative mechanical ventilation strategies for one-lung ventilation. Best Pract Res Clin Anaesthesiol. 2015; 29(3): 357-69.
- Bellamy EA, et al. Bleomycin-related lung damage: CT evidence. Radiology. 1985; 156(1): 155-8.
- Luis M, et al. Intraoperative respiratory failure in a patient after treatment with bleomycin: previous and current intraoperative exposure to 50% oxygen. Eur J Anaesthesiol. 1999; 16(1): 66-8.
- Cohen E. Management of one-lung ventilation. Anesthesiol Clin North Am. 2001; 19(3): 475-95.
- Senturk M, M Orhan Sungur and Z Sungur. Fluid management in thoracic anesthesia. Minerva Anestesiol. 2017; 83(6): 652-659.
- Lumb, A.B. and P. Slinger, Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology. 2015; 122(4): 932-46.
- Cheatham ML and JT Promes. Independent lung ventilation in the management of traumatic bronchopleural fistula. Am Surg. 2006; 72(6): 530-3.
- Levine D, T Carmichael and PC Laussen. Independent lung ventilation in a patient with complex congenital heart disease. Respir Care. 2002; 47(6): 688-92.
- Ender J, et al. High-frequency jet ventilation as an alternative method compared to conventional one-lung ventilation using double-lumen tubes during minimally invasive coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2010; 24(4): 602-7.
- Senturk M, et al. [A comparison of the effects of 50 % oxygen combined with CPAP to the non-ventilated lung vs. 100 % oxygen on oxygenation during one-lung ventilation]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2004; 39(6): 360-4.
- Jung SM and CK Cho. The effects of deep and light propofol anesthesia on stress response in patients undergoing open lung surgery: a randomized controlled trial. Korean J Anesthesiol. 2015; 68(3): 224-31.
- 19. Ishikawa S and J Lohser. One-lung ventilation and arterial oxygenation. Curr Opin Anaesthesiol. 2011; 24(1): 24-31.

