Review Article - Volume 2 - Issue 6
Clinical features and short-term results of surgical treatment of retroperitoneal extra-organ tumors
Avs Gayratovich Ulmasov1; Toru Aoyama2*; Mirzhalol Dehkanovich Juraev3; Bustonoy Sobirovna Esankulova¹; Junichi Sakamoto4; Jasur Alimdjanovich Rizaev¹; Sobirjon Ergashevich Mamarajabov
1Department of Oncology, Samarkand State Medical Institute, Samarkand, Uzbekistan.
2Department of General Surgery, Yokohama City University, Kanagawa Cancer Center, Yokohama , Japan.
3Department of Abdominal Surgery, Republican Specialized Scientific-practical Center of Medical Center of Oncology and Radiology of Samarkand Branch, Samarkand, Uzbekistan.
4Department of Palliative Care Medicine, Tokai Central Hospital, Kakamigahara, Japan.
Received Date : Oct 31, 2022
Accepted Date : Nov 25, 2022
Published Date: Dec 18, 2022
Copyright: © Toru Aoyama 2022
*Corresponding Author : Toru Aoyama, Department of General Surgery, Yokohama City University, 22-2 Seto, Kanazawa-ku, Yokohama, Kanagaw
Email: t-aoyama@lilac.plala.or.jp
DOI: Doi.org/10.55920/2771-019X/1325
Abstract
Aim: A retrospective review of the clinical data was performed with the aim of improving surgical treatment of retroperitoneal extra-organ tumors (RETs).
Method: Patients diagnosed with RETs between 2017 and 2021 were included. Patient characteristics, tumor histopathology, and details of surgical treatments were reviewed and summarized.
Results: Three hundred nine patients diagnosed with RETs were identified.Among them, 254 patients underwent surgical intervention. One hundred eighty-eight patients were younger than 60 years of age. In 52.9% of cases, combined organ resection was required. Twelve (5.8%) patients required combined resection of great vessels, while 10 patients (4.8%) underwent resection of the ureter. In 16 patients, intraoperative diffuse bleeding from the tumor bed occurred during the mobilization and removal of the tumor, resulting in death due to disseminated intravascular coagulation. The five-year survival rate after radical surgery was 41.3%.
Conclusions: RETs require combined resection of the adjacent organs and blood vessels. The surgical team must have techniques to deal with intraoperative bleeding and reconstruction of the resected organs and vessels. The achievement of long-term survival justifies the resection of the tumors in cases that require combined organ resection.
Keywords: Sarcoma; retroperitoneal; soft tissue; combined resection.
Introduction
Retroperitoneal extra-organ tumors (RETs), in general, are rare tumors which histopathological characteristics and biological behavior that can be considered benign or malignant. They originate from various tissue elements located in the retroperitoneal space [1]. Approximately 70–80% of RETs are malignant; however, these only account for 0.1–0.2% of all malignancies [2]. The classification of RETs can be based on the type of tissue origin (mesodermal, ectodermal, or embryonic remnants) [3]. These classifications are associated with microscopic features, the degree of differentiation, and biological potential. The diagnosis and treatment of RET is clinically challenging. Retroperitoneal tumors are diagnosed by radiological methods and confirmed by histology [4]. The diagnostic workup includes contrast X-rays of the abdominal organs, magnetic resonance imaging (MRI), computed tomography (CT), and ultrasound. The evaluation of tumor extension to other organs or vessels is important for planning of surgery. RETs of mesenchymal origin are characterized by rapid and aggressive growth. More than 30% of RETs have metastasis to other organs at the initial diagnosis, suggesting an aggressive nature.However, more than 60% of cases are accompanied by false-positive information on the degree of tumor invasion to adjacent organs and structures, particularly in the main vessel [5]. Thus, the extent of resection should be re-evaluated and modified after intraoperative assessment.
Patients and Methods
Patients with Ethical considerations: Patients diagnosed with RET, who were managed at the Department of Abdominal Surgery of Republican Specialized Scientific-practical Center of Medical Center of Oncology and Radiology of Samarkand Branch were enrolled in this survey. This study was approved by the Institutional Review Board (IRB) of Samarkand State Medical Institute.
Results
Patient characteristics
Three hundred nine patients diagnosed with RET were identified. At the time of the examination, 55 patients were assessed to have a tumor that was either not resectable or they had concomitant pathologies that were contraindications to surgical treatment, and therefore they were transferred to the palliative care unit. Two hundred fifty-four patients underwent surgery, 208 (81.9%) of whom underwent resection of the tumor. The remaining 46 patients (18.1%) received exploratory laparotomy alone. Among the patients who underwent resection, 56 (22.0%) patients had benign tumors and 198 (78.0%) had sarcomas. One hundred forty patients (55.1%) were male, and 114 patients (44.9%) were female. The age of the patients ranged from 16 to 77 years; the majority (188 patients; 74.0%) were younger than 60 years of age. The pathological classifications of the resected tumors were mesodermal origin in 172 (82.7%), ectodermal origin in 28 (13.5%), and embryonic remnant origin in 8 (3.8%) patients.
Diagnostic imaging modalities
Multi-slice (MS) CT with contrast media was often used to diagnose the tumor involvement of the inferior vena cava and aorta, and visceral arterial branches. Diagnostic laparoscopy was performed in order to clarify the tumor involvement of the adjacent structures, and to determine resectability, except in the cases of giant tumors that hampered laparoscopic exploration.
Features of the resected tumors
The average tumor size (in greatest dimension) was 20.2 cm. Among patients who received a full assessment, the RET was localized in one anatomical areain 58 patients (27.8 %), extended to two anatomical sitesin 117 patients (56.2%), and extended to three or more anatomical areasin 33 (15.8%) patients.
Surgical treatment of tumors
Surgical treatments included radical resection (n=135; 64.9%), palliative surgery (n=56; 26.9%), and cytoreductive surgery (n=17; 8.2%). In our study, 110 patients (52.9%) required combined surgical intervention. The resected organs included the small and large intestine, mesentery, liver, spleen, kidneys, ureters, abdominal walls, great vessels, ovaries, bladder, pancreas and other organs. In 12 (5.8%) cases, tumor invasion was extended to great vessels (invasion of the iliac arteries [n=3], invasion of the abdominal aorta [n=4], and invasion of the inferior vena cava [n=5]).In the three patients with tumor invasion of the iliac arteries, resection of the common and the external iliac artery with iliac-femoral prosthetic repair was performed (Figure 1).
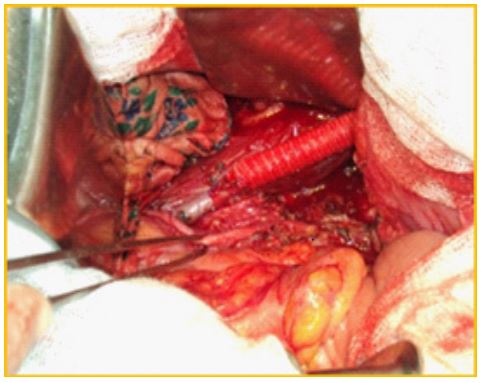
Figure 1: Resection and iliac-femoral prosthesis repair during germination of the tumor to the external iliac artery.
In 2 of 4 cases with aortic invasion, resection of the aorta with prosthetic repair was performed, while 2 case underwent plastics of the aortic wall .In all five cases with tumor invasion of the inferior vena cava (two cases above the renal veins and three cases of below renal veins), the tumor was resected with the invaded inferior vena cava plastic of the vessel walls. Ten patients (4.8%) underwent ureteral resection (length of resection: 2–10 cm); the right ureter was resected in 6 cases. In 5 cases, it was possible to overlay uretero-ureteral anastomosisdue to mobilization of the ureter. In 3 other cases, when the ureteral defectwas 8–10 cm, we developed unique ways to restore the defect. In the event of an 8-cm defect,the right side of the appendix was cut off from the cecum with preservation of the mesentery, using mobile the ileocecal angle, the ureter defect was restored using the appendicular appendage to perform appendix-ureteral anastomosis (Figure 2). Postoperative complications associated with ureteral plasticwere observed in1 of 8 patients who underwent ureteral resection (12.5%) after formation of the ureter, ureteralanastomosis for defectsof up to 3.0 cm, long-term period of up to 3 years in 2 (25%) patients had slight narrowing in the anastomotic site with the development of I-degree hydronephrosis and delivered a ureteral stent. In the remaining7 patients, ureteralpatency of anastomoses was estimated to be very good.
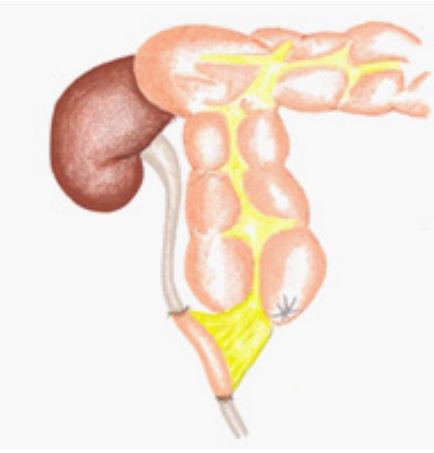
Figure 2: Replacement of the ureteral defect with appendicular appendage.
In 2 cases with a 6-cm ureteral defect, full mobilization the left kidney with renal artery, vein and the proximal end of the ureter was performed. Accordingly, it was necessary to movethe whole left kidney down by 6 cm and to perform uretero-ureteralanastomosis with nephropexy. In the third case, there was a similar situation with a 5-cm ureteral defect. The ureteral continuity was restored with mobilization of the right corner of the bladder (Figure 3).
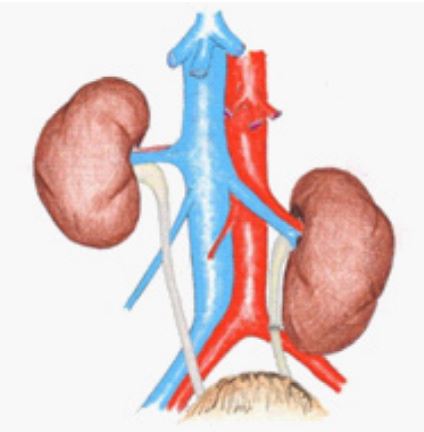
Figure 3: Bringing down the left kidney for restoration of ureteral defect.
Complications
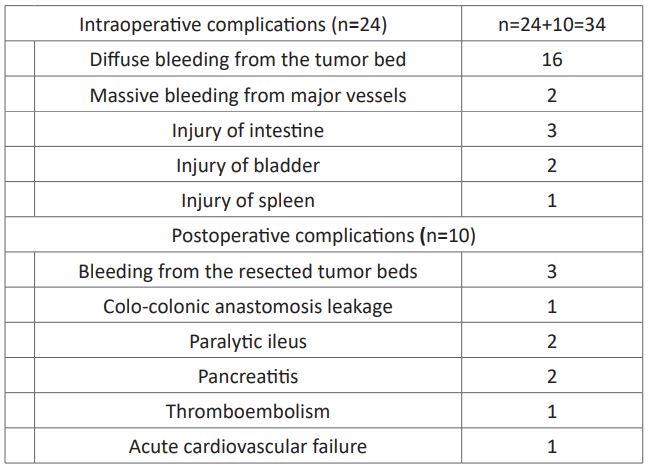
Intraoperative complications were detected in 24 (11.5%) patients, and postoperative complications were detected in 10 (4.8%) patients (Table 1). The intraoperative complications included diffuse bleeding from the tumor bed during mobilization and removal of the tumor (n=16) and massive bleeding as a result of damage to major vessels (n=2). Postoperative complications included bleeding from the resected tumor bed, external colonic fistula due to colo-colonic anastomosisleakage, and paralytic ileus. Intraoperatively one patient died from acute cardiovascular failure due to continuous bleeding and disseminated intravascular coagulation syndrome. During the postoperative period 5 patients died (intra-abdominal bleeding [n=2], thromboembolism [n=1], myocardial infarction [n=1], and relaparotomy to close the intestinal fistula [n=1]).
Table1: Intraoperativeand postoperative complications.
Long-term outcomes
In the present study, 56 patients (41.3%) achieved 5-year survival after radical surgery, while 7 patients (9.6 %) achieved 5-year survival after non-radical treatment. Recurrence within 5 years after radical surgery was observed in 82 patients (60.8%). Recurrence was observed in 15 patients with benign tumors (26.3%) and 146 patients (73.7%) with malignant tumors. In more than half (52%) of these cases recurrent disease developed within 18 months after surgery.
Discussion
In the present study, we presented the clinical features of RETs and the short-term results of the treatment of RET in our institutes. We found that the RETs could form a huge mass and invade the adjacent organs, necessitating the combined resection of the involved organs or vessels and ducts. The present study also demonstrated that intraoperative hemorrhage is a frequent complication during surgical treatment for RETs. The most crucial moment of the operation is the selection of the tumor and the so-called "trial mobilization of tumor", during which tentative revision is cautiously carried outto assess the connection of the tumor with surrounding organs and vital structures, imposing a provisional ligature for large vessels, and in principle solves a question about the volume and nature of surgical intervention [5]. At such a time during the operation, rough dislocation of the tumor into the woundis unacceptable, even when the tumor is mobilized. The famous American surgeon PH. Sugarbaker (1992) advised that surgeons adhere to a "centripetal direction of dissection", wherein "... the surgeon must repeatedly dissect the tumor in a circular manner. Major arterial vessels can be resected with the replacement of the defect with an allograft. The lower hollow inferior vena cava below the renal veins may be tied (if the only obstacle to radical removal of the tumor is its involvement in the process). According to different authors [6], surgical resection was carried out in 41% of cases, and 59% of cases were followed up with imaging. According to other authors [7], postoperative morbidity and mortality rates were 31 and 3%, respectively. The most serious complications include bleeding (without injury of the large vessels or as a result of injury of large main vessels), and the consequences of massive blood transfusion (fibrinolysis, DIC). In our practice, diffuse bleeding occurred in 16 cases. Attempts to coagulate and stitch the wound bed were unsuccessful. To successfully stop bleeding, we used tight tamponing of the tumor bed with a meter-long swab, depending on the diameter of the tumor bed, the ends of tampons were sequentially placed on the front abdominal wall through counter puncture and labeled. Tampons were left in place for 6-7 days, depending on the reaction of the organism and the emergence of sliming. Tampons were removed sequentially, first the most superficial, followed by the removal of those in the middle, and finally the lowermost tampons. Out of the 16 patients treated with tight tamponade, one patient died as a result of disseminated intravascular coagulation (DIC) syndrome. In the other 15 patients bleeding stopped after tamponade. Tampons, when indicated, were removed at 6-7 days under general anesthesia. No complications were observed in these patients.
Conclusions
In summary, to date, no standard operating procedures for RETs have been reported. RETs are often associated with severe intraoperative and postoperative complications. The surgical team must be able to control intraoperative bleeding and reconstruct the resected organs and vessels. These operations are only possible with highly professional resuscitative-anesthetic service and adequate logistical support.
Acknowledgments
This study is supported, in part, by the nonprofit organization Epidemiological and Clinical Research Information Network (ECRIN) and LLC ECRIN-2.
Conflicts of interest: The authors declare no conflicts of interest in association with the present study.
Funding source: None.
Ethical Considerations:
This study was approved by the Institutional Review Board of the Samarkand State Medical Institute (ID:131-U008-89-1-4).
References
- 1.Sokolov M, Velev G, Maslyankov S, Toshev Sv , Angelov K, Gribnev P, et al. Primary retroperitoneal extra-organ tumors (pret) - surgical tactics. Khirurgiia (Sofiia). 2015; 81(1): 4-10.
- Sassa N. Retroperitoneal tumors: Review of diagnosis and management. Int J Urol. 2020; 27(12): 1058-1070.
- Osman S, Lehnert BE, Elojeimy S, Cruite I, Mannelli L, Bhargava P, Moshiri M. A comprehensive review of the retroperitoneal anatomy, neoplasms, and pattern of disease spread. Curr Probl Diagn Radiol. 2013; 42(5): 191-208.
- Wee-Stekly W, Mueller MD. Retroperitoneal Tumors in the Pelvis: A Diagnostic Challenge in Gynecology. Front Surg. 2014; 1-49.
- Ulmasov F, Djuraev M, Khudoyorov S. Diagnosis and treatment of the retroperitoneal extra-oraganic tumors. The Advanced Science Journal. 2015; (4): 123-12.
- Sassa N. Retroperitoneal tumors: Review of diagnosis and management. Int. J. Urol. 2020; 27: 1058-1070.
- Malinka T, Nebrig M, Klein F, Pratschke J, Bahra M, Andreou A. Analysis of outcomes and predictors of long-term survival following resection for retroperitoneal sarcoma. BMC Surg. 2019; 19.

