Research Article - Volume 2 - Issue 6
Endocrine disruptors and human fertility
Ekpa Emmanuel Aduojo1*; Bello Racheal Oluwafunmilayo1; KomolafeMuhibat Bolanle1; Hassan Madinat1; Okereafor Stella2; Ajefu Christiana Ajuma3
1Faculty of Science, Biology Unit- Airforce Institute of Technology Kaduna, Nigeria.
2Faculty of Science, Department of Chemistry- Airforce Institute of Technology Kaduna, Nigeria.
3Applied Entomology and Parasitology Research unit, Department of Animal Biology-Federal University of Technology Minna, Nigeria.
Received Date : Oct 31, 2022
Accepted Date : Nov 28, 2022
Published Date: Dec 20, 2022
Copyright:© Ekpa Emmanuel Aduojo 2022
*Corresponding Author : Ekpa Emmanuel Aduojo, Faculty of Science, Biology Unit- Airforce Institute of Technology Kaduna, Nigeria.Tel: 07036135834
Email: a.ekpa@afit.edu.ng; ekpa.emmenauel@gmail.com
DOI: Doi.org/10.55920/2771-019X/1326
Abstract
Endocrine disruptors (EDs) or simply endocrine disrupting chemicals (EDCs) refer to any substance which may mimic or interfere with the normal functions of the endocrine system. They are found in everyday products like plastic bottles and containers, liners of metal food cans, detergents, flame retardants, food, toys, cosmetics, pesticides, and virtually every available human consumables. Due to the fact that most of them are slow in degradation or even completely non- biodegradable, they constitute great risk to the ecosystem. It is becoming increasingly difficult to avoid exposure to man-made endocrine disrupting chemicals (EDCs) and environmental toxicants. This escalating yet constant exposure is postulated to partially explain the concurrent decline in human fertility that has occurred over the last 50 years. Controversy however remains as to whether associations exist, with conflicting findings commonly reported for all major EDC classes. The primary aim of this current review therefore is to identify and peer-reviewed evidence regarding the effects of environmentally-relevant EDCs on adult male and female fertility during the critical preconception period on reproductive hormone concentrations, gamete and embryo characteristics. Generally, sub-fertile individuals or couples exhibit higher EDC concentrations, endorsing a positive association between EDC exposure and sub-fertility. Finally, it will highlight future research focus as well as government, industry and social awareness strategies required to mitigate the negative effects of EDC and environmental toxicant exposure on human fertility and fecundity.
Keywords: Endocrine disruptors; human fertility; fecundity; exposure; environment.
Introduction
An Endocrine Disrupting Chemical (EDC) is defined by the United States Environmental Protection Agency (EPA) as, “an exogenous chemical substance or mixture that alters the structure or function(s) of the endocrine system and causes adverse effects at the level of the organism, its progeny, and populations or (sub)populations” [1,2]. More than half a century now, there have been a general observation on increase in infertility issues especially among young couples which some even attribute to witchcraft and other diabolical reasons in this part of the world. Declining sperm counts, irregular monthly period among women of reproductive age, earlier puberty in girls, and genital malformations in people are some of the signs witnessed among groups reporting infertility challenges. Concurrently, the annual global production of plastics, which contain endocrine-disrupting chemicals (EDCs), has grown from 50 million tons to about 300 million since the 1970s and continue to increase by the day [3]. Scientific research and major findings are now beginning to reveal the connection between these major changes and inability of humans to procreate. In some ways, it’s not surprising that EDCs cause unfortunate consequences since human reproductive processes are similar to those of other biological species. Many pest-control chemicals designed to harm pest reproductive systems also damage human’s reproductive systems.Some hormones are known to be critical to reproduction like the sex hormones in women (estrogen), androgens (testosterone) in men, and hormones secreted by the pituitary gland and hypothalamus in both genders. EDCs block connections between these hormones and their receptors, or they mimic hormonal activity, thereby misleading a hormone receptor into a wrong action [3,4]. Either way, EDCs interfere with the normal function of hormonal systems. For example, estrogenic EDCs (chemicals that bind to and activate estrogen receptors) are the best studied endocrine disruptors among many others. It is pertinent to state here that globalization/Industrialization and the development of technology has made lives easy in diverse ways in this twenty first century, but it has also exertednegative effects on our health and general well-being. Reproductive system is the one mostly affected by these modern living conditions and environmental factors.
These EDCs are extremely heterogeneous and can be divided into three groups; Pharmaceuticals (e.g., diethylstilbestrol, ethinyloestradiol, naproxen, acetaminophen),Natural and synthetics hormones-(e.g., phytoestrogens, 3-omegafatty acids; synthetic such as oral contraceptives) and Environmental EDCs (e.g., polycyclic aromatic hydrocarbons, polybrominateddiphenyl ethers heavy metals, pesticides, detergents, plasticizers, solvents, dioxin and cosmetics) [5]. EDCs have been by far the biggest focus due to their widespread use and wide exposure. The major route of human exposure to these chemicals is through ingestion of contaminated water and food (e.g., meat, fish, dairy products, and vegetables), via inhalation, and through the skin. These chemicals are easily released into the environment for example through leaching into the soil and water. Some EDCs (such as some organochlorine pesticides, polychlorinated biphenyl, bisphenol-A, phthalates, heavy metals) are known as persistent organic pollutants due to their high lipophilicity. These substances pass into the systemic circulation, can be metabolized to compounds that are more toxic than the parent chemicals, and are potentially eliminated through pathways such as urine, semen, and breast milk [6]. EDCs also include different substances found in industrial compounds, children’s products (containing lead, phthalates, cadmium), food contact materials (e.g., bisphenol A, phthalates, linings of cans, or plastic bottles containing phenol), pesticides, and chemical substances widely used in cosmetics such as ultraviolet (UV) filter constituents, and parabens, as well as several heavy metals, polybrominated diphenyl ethers that are flame retardants used in agriculture, and many household and industrial products.
Healthy fertility rates depend on viable eggs in women and plentiful sperm in men. Research studies have linked EDCs to negative impacts on both.For example, BPA changes the neuroendocrine pathways fundamentally in reproductive health. Other culprits include PBDEs, used in products from flame retardants to electronics, and phthalates, which are commonly used to increase the flexibility of plastic and vinyl. There’s also evidence that EDCs induce changes to “germ cells,” the precursors to sperm and egg cells [7]. BPA exposure during a woman’s reproductive years has been shown to compromise embryo implantation. In a study in Denmark for instance, women under 40 working in the plastics industry were found out to be seeking fertility assistance than unexposed women of the same age.For men, sperm counts in certain regions of the world including the United States have declined by as much as 50 percent over the last 50 years.Most EDCs have the potential to significantly affect the development of the steroid hormone dependent human reproductive system. EDCs can interfere with the normal secretion, synthesis, production, metabolism, transport, or effect of hormones. EDCs can also alter cellular processes by different mechanisms, by binding to steroid hormone nuclear receptors and activating genomic and non-genomic pathways, activating ion channels, inducing pro-inflammatory cytokines and chemokines, promoting oxidative stress, and altering cell proliferation and differentiation [8]. EDCs can indirectly produce an estrogenic response by a number of different mechanisms, such as increasing estrogen synthesis (e.g., peroxisome proliferators inducing aromatase activity, thus increasing circulating estradiol levels), facilitating estrogen receptor binding, or altering the estrogen ratio. Estrogens are a group of chemicals of similar structure primarily responsible for female reproduction but the existence ofestrogen in men has also been reported for over 90 years. However, our knowledge of the general role of estrogens in the male reproductive and non-reproductive organs is clearly far behind than that in females. In addition, exposure to exogenous estrogens, especially developmentally, has recently been shown to have deleterious effects on the male reproductive system [9]. It is well known that chemicals interfering with hormonal pathways can seriously affect human reproductive disorders such as infertility, endometriosis, breast cancer, testicular cancer, poor sperm quality, and/or function [10]. A growing body of scientific evidence indicates that reproductive health, and ultimately reproductive capacity, is under pressure globally. Unfortunately, relatively few studies have addressed the impact of environmental exposures on human reproductive function. It has been reported that the number of families applying to infertility clinics to have a child with assisted reproductive techniques has increased significantly in recent years [11].
Infertility is defined as “a disease characterized by the failure to establish a clinical pregnancy after 12 months of regular and unprotected sexual intercourse.” It affects 10–15% of all couples and varies between countries and geographic regions. Idiopathic infertility accounts for approximately 44% of male infertility cases and is the most common individual diagnosis [12]. EDCs can also affect the duration of fertility. In girls, early life exposure to DDT may contribute to an earlier onset of puberty; once they become adults, this exposure may also lengthen menstrual cycles and accelerate menopause. Lead, another reproductive toxicant, may shorten a woman’s reproductive lifespan. Even at low levels, lead changes reproductive hormones in pre-pubescent girls and healthy premenopausal women. A recent study linked prenatal exposure to EDCs used in hydraulic fracturing (fracking) to adverse reproductive and developmental outcomes in female mice. Exposure to EDCs has been linked to structural and functional impairments of reproductive systems. In wild American alligators in Florida, exposure to a DDT-like pesticide caused genital and reproductive malformations-a phenomenon that was later seen in a variety of animal species. In people, EDCs have been linked to undescended testicles and urethra defects in men and endometriosis and fibroids in women. Ovarian cysts have been associated with higher amounts of chemicals such as BPA [13]. This current review will examined the detrimental effects of EDCs exposure on male/female fertility, by providing an overview of reported literature studies so as to educate all those concerned and possibly make future projections.
Types and Prevalence of Commonly known EDCs
As information (and misinformation) proliferates in the media about endocrine-disrupting chemicals (EDCs), so do questions about where they are found and how humans are exposed to them through eating, drinking, breathing, or touching. Simply put, how do our normal senses come in contact with these very harmful substances? Answers to these questions are not always simple considering the array of chemicals that are constantly discovered to have endocrine disrupting effects. There are nearly 85,000 man-made chemicals in the world, many of which people come into contact with every day. Only about one percent of them have been studied for safety; however, 1,000 or more of these chemicals may be EDCs based on their probable endocrine-interfering properties. The list of EDC’s keep growing everyday as new materials are been studied and added to the already known ones. A few of them are discussed below.
Brominated flame retardants (BFRs) used in electronics, clothing, and furniture such as sofas and mattresses to reduce flammability. Unfortunately, these chemicals also have been linked to abnormal hormone function in the thyroid, which plays a critical role in fetal and childhood development. Adding to the risk of exposure, BFRs often migrate out of their products over time where they may contaminate household dust and food.
Polychlorinated biphenyls (PCBs) are used in hundreds of industrial and commercial applications due to their non-flammability, as well as chemical stability and insulating properties. Although the EPA banned their manufacture in the United States in 1979, PCBs are still present in insulation, electrical equipment, caulking, oil-based paint, and more, and do not break down readily. In addition to being a long-acknowledged toxicant, PCBs are EDCs. As a class, they have the strongest and longest-known associations with neurological disorders.
Phthalates interfere with the production of androgen (testosterone), a hormone critical in male development and relevant to females as well. Phthalates are used in hundreds of products, including many food and beverage containers and plastic wraps. People are exposed to these EDCs when they leach into foods or are released when containers are microwaved. Many companies have voluntarily removed phthalates from their products and advertise them as “phthalate-free”. Other plastic containers, which contain phthalates, have the number "3" and “V” or “PVC” in the recycling symbol for public awareness. The European Union has restricted some members of this EDC class since 1999, and the United States has similarly restructure their use since 2008. Phthalates are usually identified on product labels by the specific compound: The eight most common are; Lead – long acknowledged as a neurological toxicant-has also been linked with adverse female reproductive functions in animal, in-vitro, and human epidemiological studies. While lead has been banned in house paints, dishes, and cookware in the United States since 1978, this EDC may still be found in a product’s paint -especially in products manufactured in countries which still allow lead-based paint-and in plastics where lead is still allowed for softening and stabilizing against heat. Studies by the International Persistent Organic Pollutants Elimination Network (IPEN), a non-governmental organization working for safe chemical policies in the developing world, reported lead in 18 percent of children’s products in Russia and surrounding nations, 15 percent in the Philippines, and 10 percent in five cities in China [14]. Cadmium is a natural element used in batteries, pigments, plastic stabilizers, alloys, and coatings. It has in recent years fallen under increased regulation as a carcinogen and pollutant. Cadmium may also be an EDC; research suggests a link to a wide range of detrimental effects on the reproductive system.
Bisphenol A (BPA) is one of the best known and most pervasive group of EDCs. In humans, it is linked to reduced egg quality and other aspects of egg viability in patients seeking fertility treatment.Although BPA was banned in children’s products such as baby bottles, it’s still used in many water bottles and plastic containers and in the epoxy resins that protect canned foods from contamination. In these products, BPA leaching is enhanced by heating or reheating (such as in a microwave), or exposure to sunlight or acidic foods (such as tomatoes). EDCs are gradually being regulated and banned in children’s toys, games, and accessories such as baby bottles. However, products that are older, manufactured outside of the United States and European Union, or battery-operated may be of particular concern. Many pesticides are designed to be toxic to pests’ nervous or reproductive systems and may act by disrupting endocrine systems. Such chemicals are also EDCs because of the similarities between insect and animal endocrine systems. They include:
Chlorpyrifos, an insecticide used in commercial agriculture.It is a potent neurotoxicant that causes developmental delays, attention problems, and ADHD in children. It accumulates in soil, water, food, and air, as well as in buildings. That’s why the United States banned its residential uses in 2000, and the effect was immediate: children’s blood levels of chlorpyrifos in New York declined significantly in one year and were reduced to less than half in two years.
DDT, one of the best-known pesticide EDCs, was used extensively worldwide until it was banned in the 1970s by several countries, including the United States and European Union nations. It remains in use in regions such as India and Africa to fight insect-borne diseases. Emerging evidence suggests that exposure to this neurotoxin might be associated with breast cancer, preterm birth, early pregnancy loss, reduced semen quality, disrupted menstruation, and problems with lactation.
Atrazine, a widely-used herbicide, has been shown to affect the hypothalamus and pituitary glands. Some studies have also proposed causal relationships between glyphosate, used to kill weeds on lawns and farms, and obesity, behavioural, and cognitive disorders.
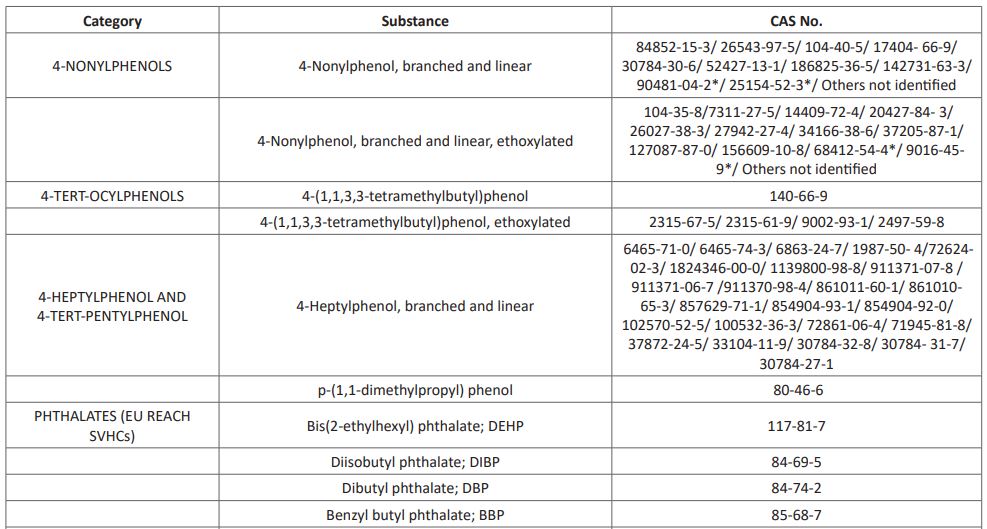
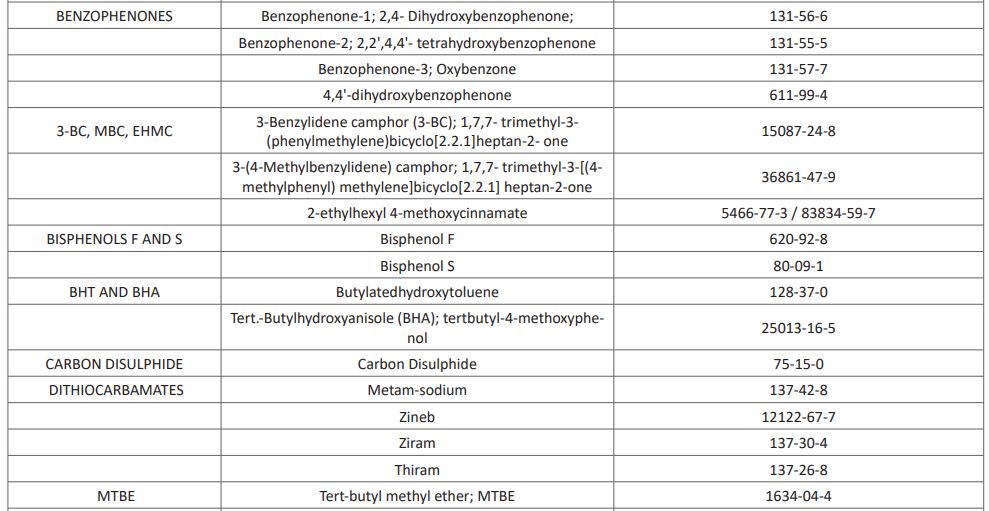
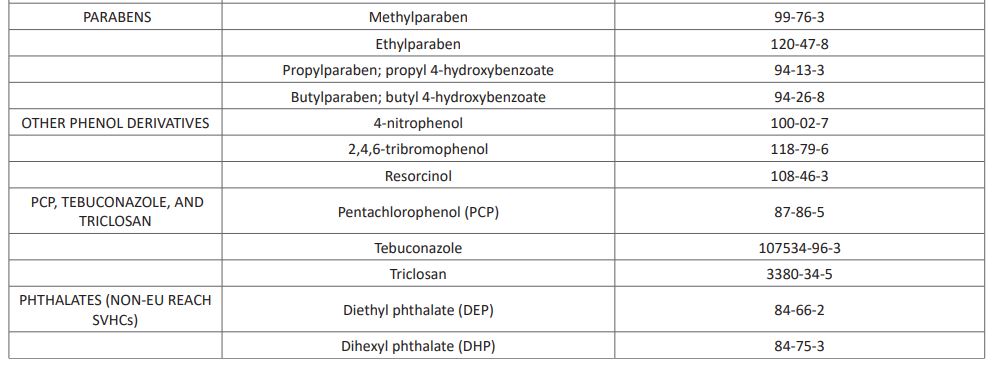
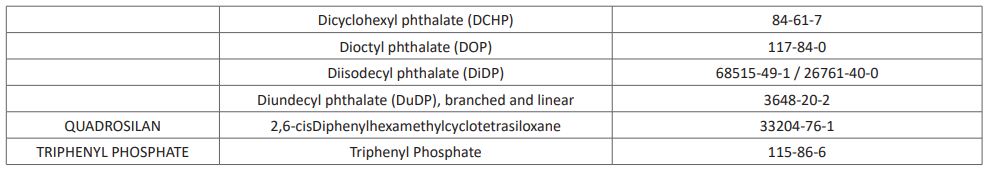
Per-and polyfluoroalkyl substances (PFAS) are man-made chemicals used as oil and water repellents and coatings for common products including cookware, carpets, and textiles. These endocrine-disrupting chemicals do not break down when they are released into the environment, and they continue to accumulate over time [15]. Other known EDCs recognized as at 2017 by the United Nations (UN) are listed in (Table 1). This list however keeps growing from time to time as new substances possessing characteristics of EDCs are continuously emerging and been added to the list.
Table 1: UN List of Identified EDCs
The list includes 45 chemical substances (as end of July 2017). The chemicals are claimed to have gone through at least one "thorough scientific assessment.
Sources:
- https://chemicalwatch.com/68296/un-publishes-list-of-identified-endocrine-disruptors
- https://www.unenvironment.org/explore-topics/chemicals-waste/what-we-do/emerging-issues/scientific-knowledge-endocrine-disrupting
- https://wedocs.unep.org/bitstream/handle/20.500.11822/25634/edc_report2.pdf?sequence=1&isAllowed=n
EDCs and Male infertility
Infertility, which is currently defined as the inability to conceive after one year of unprotected intercourse, has a global prevalence of 9%, ranging from 3.5% to 16.7% in developed countries, compared to 6.9-9.3% in developing countries. A multi-centre study conducted by the World Health Organization among infertile couples shows that the cause was predominantly female in 38% of the cases and primarily male in 20%, while 27% of couples had both male and female abnormalities, and no evident cause was identified as for the remaining 15%. Since the mid-twentieth century, a cumulative number of studies report an increasing incidence of human reproductive diseases and consequent decline in reproductive function, in several locations and amongst different populations. Given this relatively short time frame, genetic changes may not explain the rising infertility rates. Thus, environmental substances may be one of the factors responsible for the observed geographical and population differences in the incidence of infertility especially in the male gender [16]. The psychological, social, and economic implications of reduced male sexual ability to have children are often unimaginable and goes beyond individuals to entire families and society at large. Studies in Men from different part of the world over the past 10 years have shown a drop in semen quality. EDCs affect the maturation, function, and viability of sperm by acting directly on the sperm or altering the function of the epididymis as well as the sperm’s capacity to fertilize an egg [17]. In normal human males, the number of sperm is close to what is normally needed for fertility. While acute exposure can cause significant changes in spermatogenesis, it appears to occur with low-dose, chronic exposures to EDCs that impair spermatogenesis. Therefore, even a little decrease in daily sperm production can lead to infertility. Semen parameters are used to measure sperm quality and they are very important because they can be used to predict male reproductive health [18].
It has been estimated that about 15% of couples globally are infertile and half is attributed to the male factor. Inability to pregnant a woman by men is considered as primary cause of infertility in 20% of couples and a contributing factor in 30–40% of cases. Some of these causes may be due to changes in the hypothalamic-pituitary-gonadal (HPG) axis or by direct effects on sperm and other semen parameters [19]. Men with sperm parameters below the values specified in WHO are considered to have male factor infertility. The most important of these are low sperm concentration (oligospermia), poor sperm motility (asthenospermia), and abnormal sperm morphology (teratospermia). Other factors less correlated with infertility include semen volume and other seminal markers.Sperm function is affected by reactive oxygen species (ROS) produced during the metabolism of these chemicals, which is another possible effect of infertility including EDCs. Oxidative stress plays an important role in the mechanism of male infertility. Oxidative stress is a balance between the production of ROS and the natural antioxidant defense of semen. Increased ROS levels can be due to many factors such as environmental pollutants and lifestyle factors.The effect of EDCs in testicles is mediated mainly by the nuclear estrogen receptors (ESR1 and ESR2) expressed by Sertoli and germ cells. These cells secrete masculinizing hormones that regulate sperm production, [20]. Because hormones tightly control the male reproductive system, anti-androgens or EDCs that mimic estrogens can interfere with spermatogenesis and have a profound effect on healthy sperm production. Men exposed to estrogenic EDCs may reduce fertility and develop female secondary sex characteristics such as gynecomastia.
Reproductive problems in males due to metal exposure are one of the most important areas of concern in toxicology. In epidemiological and clinical studies, it has been found to be associated with impaired semen quality as a result of the direct effect of heavy metals on testicular function or hormonal changes. One of the heavy metals of greatest concern is lead (Pb). Lead exposure can cause toxicity to both the male and female reproductive systems. Pbis a natural heavy metal and is regularly used in mining, smelting, refining, leaded gasoline (petrol), lead-acid batteries, paints, jewellery, children’s products, and many other products. The general population is exposed to Pb through contaminated food, water, and inhalation of airborne Pb. Lead in seminal plasma may increase with environmental pollutions, and industrial and dietary exposure [21]. Strong evidences confirm that male infertility in metal-exposed humans is mediated via various mechanisms such as production of reactive oxygen species (ROS). It is known that smoking causes oxidative stress by increasing oxidant levels or decreasing antioxidant levels in seminal plasma. Lead (Pb) levels in blood were investigated by [22] and seminal plasma of the infertile and fertile groups were also checked in a research conducted. Findings shows that Lead (Pb) levels in seminal plasma and blood were significantly higher in infertile men than those in fertile groups. It was revealed that sperm count, motility, and morphology were significantly decreased in infertile smokers than in non-smoker infertile and fertile men. The researchers further investigated whether oxidative stress is an intermediate mediator in regulating the associations between heavy metal exposure and impaired semen quality. A significant inverse relationship was found between lead (Pb) exposure and the percentage of normal sperm morphology, and a negative correlation was detected with the sperm count and motility. Therefore, the authors postulated that unexplained male infertility may be due to increased Pb levels [23]. This same scenario plays out with almost all EDCs already mentioned before.
EDC and Female Infertility
A woman’s reproductive health is largely established during embryonic and feta development and later, during puberty. Female reproductive disorders in adulthood can originate from interference with hormone levels and function during development, as was clearly demonstrated by the “DES disaster”, where overt reproductive effects have been described in children born from women taking the synthetic oestrogen diethylstilbestrol (DES) as a drug during pregnancy. The adverse health effects include a rare form of vaginal cancer in girls, increased incidence of uterine fibroids, endometriosis, impaired fecundity and earlier age at menopause. Startlingly, reproductive effects are still apparent in women whose mothers have been prenatally exposed to DES.In the transition from (unborn) child to an adult woman, many hormonal processes are activated or reactivated. This means that basic biology of the female reproductive system is very different in the embryo, fetus, newborn, young girl, adolescent, and adult women. Consequently, the effect of EDC exposure on the female reproductive system depends on the age or life stage when exposure occurs [24]. The number of women with reproductive health problems is increasing globally too and scientific knowledge clearly points towards exposure to human-made chemicals being a contributing factor. It is clear that many chemicals in our food and the environment can disrupt endocrine processes and thereby threaten the reproductive health of humans, livestock, and wildlife globally [26]. Numerous concerns about reproductive effects in humans and wildlife originate from findings linking exposure to EDCs in the womb to testicular dysgenesis syndrome, characterised by declining sperm counts and increasing prevalence of undescended testes, testicular cancer, and urinary duct malformation in males. However, in contrast to male reproduction, we know surprisingly little about the mechanisms by which EDCs can impair female reproduction. Inevitably, therefore, the definition of ovarian dysgenesis syndrome is much more loosely encompassed in females. This incomplete knowledge about cause–effect relationships between chemical insult and disease manifestation in women is partly the reason why we lack good test methods to address this in regulatory chemical risk assessment practice.
EDCs are thought to affect women’s menstrual cycle, estrogen deficiency, infertility, and are also associated with diseases such as polycystic ovary syndrome (PCOS) and endometriosis, spontaneous abortions, birth defects, endometriosis, breast cancer, premature ovarian failure. Female are at a greater risk than men, especially with the rise in environmental estrogens. However, since research on these exposures often tends to focus on male fertility, it is unlikely that EDCs will answer questions about female fertility. Because females are relatively sensitive to estrogens and are heavily exposed to environmental estrogens, women will also be most affected by EDCs. The origin of endocrine disruption hypothesis was related to exposure to estrogens. Literature data also show that long-term and combined exposure to environmental estrogens will have an impact on female fertility. Although it has long been known that female fertility is impaired by estrogen exposure, there are limited data on whether long-term low-dose exposure to environmental pollutants with weak estrogenic effect causes problems such as infertility in women. There is little epidemiological information about trends in female infertility. Data on the effects of EDCs on the female reproductive system and fertility are insufficient. However, it has been suggested that there is a relationship between exposure to EDCs and their long-term effects. The most common direct or indirect causes of female infertility are endocrine problems. EDCs alter endocrine function through various mechanisms. One of these mechanisms is that these substances directly bind to estrogen receptors and increase aromatase activity, thereby increasing estrogen sensitivity. Another mechanism is that EDCs indirectly lead to an increase in endogenous estrogen production and exert their effects through both receptor-dependent and receptor-independent mechanisms through their effects on gonadotropin-releasing hormone. Both mechanisms result in altered ovarian function by altering endocrine signaling with several processes in ovary and the other reproductive organs.
Good reproductive health is important for the health and well-being of women and, if they wish to conceive, for the health of their children and future generations. Estimations show that up to one in six couples in Western countries experience problems conceiving or carrying pregnancy to term; however, the prevalence of subfertility varies widely between studies due to differences in study design and classifications. A woman’s fertility decreases as she gets older, but even during her most fertile years, lifestyle choices and external factors can affect her chances of having a healthy baby. Ovulation disorders account for infertility in about 1 in 4 infertile couples. Examples of ovulation disorders are polycystic ovary syndrome (PCOS) and primary ovarian insufficiency (POI) also known as premature ovarian failure (POF). A disrupted hormone balance is a clear manifestation of these disorders and often appears to be an underlying mechanism in the aetiology. Therefore, it is not surprising that exposure to EDCs is associated with reduced female fertility and ovarian disorders. For example, epidemiological studies show that women with PCOS have higher levels of bisphenol A (BPA), and perfluorooctanoic acid and perfluoro-octanesulfonate compared to women without PCOS. There are strong indications to link EDC exposure to POI, however, except for cigarette smoking, there is a lack of relevant human data addressing this. Furthermore, disturbances of hormone levels-e.g., by BPA-can cause menstrual cycle irregularities as well as affect the quantity and quality of available oocytes.
Female fertility depends upon a functional reproductive system, capable of producing fertilizable oocytes through folliculogenesis, a multi-stage process which requires specific endocrine signals. Both oestrogens and androgens are essential for maintaining HPG homeostasis, which is crucial for follicular development and ovulation, and ultimately female fertility. Proper differentiation of the female reproductive system is regulated by sex hormones, particularly oestrogens, but it proceeds even in their absence, being regarded as the default developmental pathway. Nevertheless, oestrogen signalling pathway overstimulation during critical developmental periods is known to result in several irreversible abnormalities. All components of the female reproductive system are prone to EDC action particularly during the developmental period. The array of female reproductive disorders where EDC have been implicated is fairly large, comprising endometriosis, disorders of the uterus, as well as disorders of the ovary, such as premature ovarian failure (POF), oocyte aneuploidy and polycystic ovary syndrome (PCOS), all of which may result in infertility [27]. Since these disorders may arise from impaired ovarian development and function, the term Ovarian Dysgenesis Syndrome (ODS) has recently been suggested as the female form of TDS. The incidence of ODS traits is growing. Data concerning EDC effects on female reproductive system and fertility are scanty, when compared to the male counterpart, in both human epidemiological studies and experimental literature. Still, a correlation between developmental EDC exposure and long-term effects is suggested. For instance, women whose mothers had high maternal serum concentration of DDT during pregnancy were found to have a significantly higher risk of infertility. In adult women, high serum concentration of EDC, such as BPA, is associated with a variety of female reproductive system disorders, including endometrial hyperplasia, PCOS, and infertility. Occupational exposure to EDC such as pesticides, plastics, and other industrial chemicals is a risk factor for female infertility. Many EDC can potentially hamper the differentiation of the female reproductive system, both in utero and after birth, by disrupting the appropriate expression of oestrogen-regulated genes. The development of the female reproductive system is regulated by the differential expression of Hoxgenes in the Müllerian duct [28]. Disruption of the precise chronological regulation of Hox genes by EDC, that either increase (e.g. BPA) or down-regulate (e.g. DES and MXC) the expression of this gene, has been shown to lead to uterine abnormalities and infertility.
Effect of Leaad (Pb) on Female fertility
Lead (Pb) is known to be one of potential female reproductive toxins. However, there are few studies on whether low Pb exposure causes female infertility compared with male infertility. Lead is a potent disruptor of adrenal and ovarian steroidogenesis and inhibits progesterone synthesis and activity in dose-dependent manner. The effects of lead on 17-β-estradiol, testosterone, and cortisol may cause stimulant effects after low-level exposure, while inhibiting effects after high-level exposure. Exposure to Pb causes impaired fertility in women, two key proteins in the function of the pituitary-ovarian axis. Both P-450 aromatase and ER-β-activity in granulosa cells of ovarian follicles have been shown to be strongly inhibited in women exposed to Pb [28]. It is known that Pb can concentrate, impair cellular processes, and lead to harmful results in terms of reproductive health. Research has found out that low blood lead level was positively associated with infertile women. It has been suggested that even low blood lead levels may be detrimental to female fertility. Another researcher investigated the association between blood concentrations of Pb and risk factors for infertile or pregnant women in Taiwan. The concentration of Pb was significantly higher in the blood of infertile women than in that of pregnant women. Particularly, frequent use of Chinese herbal medicine by infertile women has been associated with elevated blood Pb levels. It is suggested that the risk-benefit of Chinese herbal medicine intake should be evaluated by women of childbearing age. Due to increase in the female workforce of more women in Pb production in developing countries, more women are exposed to potential reproductive hazards. In a study conducted by some group of Scientists, it was shown that Pb causes reproductive toxicity and female infertility as a result of occupational exposure (lead battery plants). In this study, it was observed that the menstrual cycle, that is, the hormonal balance of female workers exposed to lead was disturbed [29]. On the other hand, it was investigated whether the urinary heavy metal excretion was associated with different factors of infertility. It was found that accumulation of heavy metals in the ovary disturbs the production of estradiol and progesterone eventually.
Conclusions
Men and women are exposed to many EDCs, including phthalates, BPA, pesticides, and persistent environmental contaminants such as PCBs and PAH on a daily basis. This calls for concern because many EDCs are known to affect normal reproduction in both gender. In this review, we summarized that these EDCs have a negative impact on male and female fertility in a variety of ways. Literature on animal studies show that in females, ancestral EDC exposure can alter litter size, alter anogenital distance, cause early puberty, disrupt estrous cycle, alter follicle numbers, cause early reproductive aging, decrease fertility, increase cysts in ovaries, alter sex ratios in pups, alter sex steroid hormone levels, and cause adenomyosis. While in males, ancestral EDC exposure can alter anogenital distance, cause early puberty, decrease fertility, cause testes disease, decrease sperm count and motility, alter sperm morphology, and alter sex steroid hormone levels. In general, it is being observed that the most important harmful effects of exposure to endocrine-disrupting environmental pollutants such as heavy metals, pesticides, and cosmetics are on the reproductive system in humans. Infertility is both clinical and social problems that affect the couple’s life, health services, and social environment. With the awareness of these important issues, factors that increase the risk of infertility can be prevented. Further toxicological studies are needed to further understand the risk and mechanisms of action of these substances on male and especially female reproductive function, and to identify and characterize new EDCs so as educate all stakeholders on measures to be taken in the future.
References
- Famurewa AC, Ugwuja EI. Association of blood and seminal plasma cadmium and lead levels with semen quality in non-occupational exposed infertile men in Abakaliki, south East Nigeria. Journal of Family & Reproductive Health. 2017; 11(2): 97-103.
- Vigeh M, Smith DR, Hsu PC. How does lead induce male infertility? Iran. The Journal of Reproductive Medicine. 2011; 9(1): 1-8.
- Sengupta P, Banerjee R. Environmental toxins: Alarming impacts of pesticides on male fertility. Human& Experimental Toxicology. 2014; 33(10): 1017-1039. [DOI: 10.1177/0960327113515504].
- Mehrpour O, Karrari P, Zamani N, Tsatsakis AM, Abdollahi M. Occupational exposure to pesticides and consequences on male semen and fertility: A review. Toxicology Letters. 2014; 230: 146-156. [DOI: 10.1016/j.toxlet.2014.01.029].
- Hara L, Smith LB. Androgen receptor roles in spermatogenesis and infertility. Best Practice & Research. Clinical Endocrinology &Metabolism. 2015; 29: 595-605. [DOI: 10.1016/j.beem.2015.04.006].
- Tang W, Wang D, Wang J, Wu Z, Li L and Huang M. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere. 2018; 191: 990-1007. [DOI: 10.1016/j.chemosphere.2017.10.115].
- Wang Q , Shen JY, Zhang R, Hong JW, Li Z, Ding Z. Effects and mechanisms of pyrethroids on male reproductive system. Toxicology. 2020; 438: 152460. [DOI: 10.1016/j.tox.2020.152460].
- Oehninger S, and Kruger TF. Sperm morphology and its disorders in the context of infertility. F&S Reviews. 2021; 2(1): 75-92. [DOI: 10.1016/j.xfnr.2020.09.002].
- Merzenich H, Zeeb H, Blettner M. Decreasing sperm quality: A global problems? BMC Public Health. 2010; 10: 24. [DOI: 10.1186/1471-2458-10-24].
- Alam MF, Akhter M, Mazumder B, FerdousA, Hossain MD and Dafader NC. Assessment of some heavy metals in selected cosmetics commonly used in Bangladesh and human health risk. Journal of Analytical Science and Technology. 2019; 10: 1-8. [DOI: 10.1186/s40543-018-0162-0].
- Endocrine Disruptors [Internet]. National Institute of Environmental Health Sciences. 2021. Available from: https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm.
- Research NC for T. Endocrine Disruptor Knowledge Base [Internet]. FDA. FDA 2009. 2021. Available from: https://www.fda.gov/science-research/bioinformatics-tools/endocrine-disruptor-knowledge-base.
- WHO | Global assessment of the state-of-the-science of endocrine disruptors [Internet]. WHO. World Health Organization; [cited 2021 Feb 8]. Available from: https://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en/
- Jones L, Regan F. Endocrine Disrupting Chemicals. In: Reference Module in Chemistry, Molecular Sciences and Chemical Engineering [Internet]. 2018. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780124095472145123.
- Schneider M, Pons JL, Labesse G and Bourguet W. In Silico Predictions of Endocrine Disruptors Properties. Endocrinology. 2019; 160(11): 2709-16
- Bergman Å. United Nations Environment Programme, World Health Organization. State of the science of endocrine disrupting chemicals - 2012 an assessment of the state of the science of endocrine disruptors [Internet]. Geneva: WHO : UNEP 2013. 2012. Available from: http://www.who.int/ceh/publications/endocrine/en/index.html.
- Lucaccioni L, Trevisani V, Marrozzini L, Bertoncelli N, Predieri B and Lugli L. Endocrine-Disrupting Chemicals and Their Effects during Female Puberty: A Review of Current Evidence. Int J Mol Sci. 2020;21(6): 2078.
- Bell MR. Endocrine-disrupting actions of PCBs on brain development and social and reproductive behaviors. CurrOpinPharmacol. 2014; 19: 134-44.
- Arora NK, Nair MKC, Gulati S, Deshmukh V, Mohapatra A, Mishra D. Neurodevelopmental disorders in children aged 2–9 years: Population-based burden estimates across five regions in India. PLOS Med. 2018; 15(7): e1002615.
- Harley KG, Gunier RB, and Kogut K. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res. 2013; 126: 43-50.
- Braun JM, Kalkbrenner AE, and Calafat AM. Impact of early-life bisphenolA exposure on behavior and executive function in children. Pediatrics. 2011; 128: 873-882.
- Shelton JF, Geraghty EM and Tancredi DJ. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE Study. Environ Health Perspect. 2014; 122: 1103-1109.
- Chen F, Zhou L, Bai Y, Zhou R and Chen L. Sex differences in the adult HPA axis and affective behaviors are altered by perinatal exposure to a low dose of bisphenol A. Brain Res. 2014; 1571: 12-24.
- Cao J, Rebuli ME and Rogers J. Prenatal bisphenolA exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol Sci. 2013; 133: 157-173.
- Monje L, Varayoud J, Muñoz-de-Toro M, Luque EH and Ramos JG. Exposure of neonatal female rats to bisphenol A disrupts hypothalamic LHRH pre-mRNA processing and estrogen receptor expression in nuclei controlling estrouscyclicity. Reprod Toxicol. 2010; 30: 625-634.
- Yu B, Chen QF and Liu ZP. Estrogen receptor and expressions in hypothalamus-pituitary-ovary axis in rats exposed lactationally to soy isoflavones and bisphenol A. Biomed Environ Sci. 2010; 23: 357-362.
- Castro B, Sánchez P, Torres JM, Preda O, del Moral RG, and Ortega E. Bisphenol A exposure during adulthood alters expression of aromatase and 5 alpha-reductaseisozymes in rat prostate. PLoS One. 2013; 8.
- Panagiotidou E, Zerva S, Mitsiou DJ, Alexis MN, and Kitraki E. Perinatal exposure to low-dose bisphenol A affects the neuroendocrine stress response in rats. J Endocrinol. 2014; 220: 207-218.
- Lauretta R, Sansone A, Sansone M, Romanelli F and Appetecchia M. Endocrine Disrupting Chemicals: Effects on Endocrine Glands. Front Endocrinol. 2019; 10: 178-50.

