Short Communication - Volume 2 - Issue 6
Synergistic effects of cannabis with gabapentin and its analog pregabalin (Lyrica®)
Nawaf Almuntashiri1; Hassan Alzahrani1; Rahma Kofiya1; M.E. Alzuhiri1; A.Y. Hamdi1; N.A. Badahdah1; Ali Almaghrabi1; Helal Zamil1; Osama Alsahafi2; Mohammad Gamaruddin3; Abdullah Mahdi4; Al-Farga Ammar5*
1Center of Poison Control and Forensic Medicinal Chemistry in Makkah, Ministry of Health, Saudi Arabia.
2Khulais General Hospital, Makkah Health Cluster, Saudi Arabia.
3Ajyad Emergency Hospital, Makkah Health Cluster, Saudi Arabia.
4Maternity and Children Hospital, Makkah Health Cluster, Saudi Arabia.
5Department of Biochemistry, College of Science, University of Jeddah, Jeddah, Saudi Arabia.
Received Date : Nov 05, 2022
Accepted Date : Dec 01, 2022
Published Date: Dec 23, 2022
Copyright:© Al-Farga Ammar 2022
*Corresponding Author : Al-Farga Ammar, Department of Biochemistry, College of Science, University of Jeddah, Jeddah, Saudi Arabia.
Email: alfergah83@gmail.com
DOI: Doi.org/10.55920/2771-019X/1329
Introduction
Cannabis indicates a group of three plants (Cannabissativa, C.indica, and C.ruderalis) having psychoactive properties. Cannabis is majorly consumed for its calming and relaxing effects. In the United States, cannabis is also indicated for use in a range of medical ailments, including glaucoma, chronic pain, poor appetite etc. Cannabis contains more than 120 constituents, known as cannabinoids. Researchers aren’t able to fully explore effects of cannabinoids [1].
Cannabidiol (CBD), one of the non-psychoactive constituent of cannabinoid, is effective as an adjunctive treatment with epilepsy in children associated with Lennox–Gastaut and Dravet syndromes. Therefore, National Institute for Health and Care Excellence (NICE), Food and Drug Administration (FDA), and European Medicines Agency (EMA) have approved the use of highly purified CBD (Epidiolex® approved recently for use in Lennox–Gastaut or Dravet syndrome in the United States of America [USA]) with the NICE and EMA specifying its use only as an choice for adjuvant treatment with clobazam in the treatment of Lennox–Gastaut and Dravet syndromes [2].
Tetrahydrocannabinol (THC) is the key psychoactive constituent of cannabis. Tetrahydrocannabinol (THC) and its metabolites (11-hydroxy-THC and 11-nor-9-carboxyl-THC) have especially long half-lives, 25–57 hours and about 5 days, respectively [3]. The endocannabinoid system has long been identified as a key therapeutic option. Either the highly purified CBD or formulations with different THC to CBD ratios (like Sativex/ Nabiximols, an oromucosal spray for the management of spasticity associated with multiple sclerosis) are being explored for other disease conditions. While some clinical trials support the use of cannabinoids for the treatment of pain, currently there is only some evidence to back the cannabinoids usage in the management of chronic pain and further exploratory clinical trials are required. In spite of this, chronic pain relief is mostly the most common ailment cited by the patients who use cannabis for medicinal effects and limited data is available about the potential pharmacokinetic drug interactions with medications prescribed to alleviate chronic pain. Cannabinol (CBN), a THC degradation byproduct, is now being studied for its analgesic properties.
Cannabidiol (CBD), THC and CBN are metabolized extensively in liver and intestine. Biotransformation of CBD is mediated primarily by CYP2C19 and, CYP3A4, to a lesser extent. Cannabidiol (CBD) can also get conjugated directly by the various UDP-glucuronosyltransferase (UGT) enzymes (such as UGT1A9, UGT2B7, and UGT2B17). Biotransformation of THC primarily depend on the CYP2C9 and CYP3A4 isoenzymes, but the UGT enzymes also play a crucial role in metabolizing the THC metabolites. Cannabinol (CBN) is mostly metabolized by CYP2C9 and CYP3A4 isoenzymes, and can undergo glucuronidation directly by the hepatic (UGT1A9) as well as extrahepatic (UGT1A7, UGT1A8, and UGT1A10) isoenzymes. Cannabidiol (CBD) is a substrate as well as an inhibitor of CYP450 enzymes and the UGT enzymes. Tetrahydrocannabinol (THC) and CBN also inhibit certain isoenzymes of the cytochrome P450 or the UGT enzymes. In terms of cannabinoid inducing activity, smoked cannabis may raise the removal of drugs metabolized by CYP1A2 enzyme, leading to their reduced concentrations and possible treatment failure.
Moreover, various in vitro and in vivo studies have revealed that CBD, THC, and CBN interact with two members (breast cancer-resistant protein [Bcrp] and glycoprotein P [Pgp]) of ATP-binding cassette family. As a result, other concomitantly administered drugs which are substrates of these transporters may have a crucial effect on their absorption and distribution. Cannabidiol (CBD) has been shown to suppress Pgp and Bcrp, in some pre-clinical studies.
Although the inhibitors are mostly substrates, various studies showed that CBD is not a substrate of Pgp and causes a downregulation in expression of Pgp. Tetrahydrocannabinol (THC) and CBN could also cause deregulation of Pgp, Bcrp, and expression of multidrug-resistant protein (MRP) 1-4. As the cannabinoids are frequently utilized as an add-on to treatment, the incidence of DDIs appears to be more conceivable. Consequently, their utilization in the treatment could impede the distribution and elimination of different medications that go through similar metabolic pathways. Regardless, fewer investigations in human beings have been reported in literature about the DDIs of cannabinoids with other drugs; few of them are case reports.
Albeit various pre-clinical investigations about the DDIs ought not be extrapolated to humans, the healthcare providers ought to know about significant DDIs leading to either therapeutic improvement, failure or toxicity. A dramatic rise in the use of cannabis over the last few years has resulted in an increase in the number of patients taking it alongside other medications. As a result of this situation, cannabinoids may be categorized as culprits or substrates based on the use of concomitant drugs, resulting in adverse events, changed exposure, clinical inefficacy etc. But there is little evidence about drug interactions of cannabis and their potential implications in terms of clinical efficacy and safety.
A drug-drug interactions (DDI) take place when a drug changes the clinical impact of other drug. These interactions might take place at both pharmacokinetic and pharmacodynamic levels. Pharmacokinetic DDIs include a drug altering the absorption, distribution and elimination of other drug administered concomitantly which can cause a change in quantity of the drug at the drug site affecting the extent and duration of drug effect. Pharmacodynamic DDIs include one drug altering the responsive or sensitivity to other drug [4].
Interaction with anticonvulsants
Antiepileptic/antiseizure medications are utilized globally to treat various ailments apart from epilepsy, for example, migraine, neuropathic pain, and bipolar disorder. The first-line choices for the management of different neuropathic pain include pregabalin and gabapentin [5]. Pregabalin or the (S)-3-(aminomethyl)-5-methylhexanoic acid, is a centrally-acting neuromodulating drug which was approved in 2004 by the United States (US) FDA for the management of post-herpetic neuralgia and diabetic neuropathic pain. It is a derivative of the inhibitory chemical messenger, gamma-aminobutyric acid (GABA). It is fundamentally associated with gabapentin and has comparable pharmacological and anticonvulsant and analgesic effects [6].
Additionally, the use of gabapentinoids together with cannabis can cause a number of harmful effects; the use of gabapentin with cannabis may cause drowsiness, dizziness, confusion, and difficulty in concentrating. Many people, particularly the elderly, may too experience impairment in judgment, thinking, and lack of motor coordination. The use of alcohol should also be avoided while using these medications. Activities which require mental alertness like driving, operating heavy machinery should also be avoided until the medications are used [7]. It is vital to let the physician know about all the drugs that are being consumed concomitantly, including vitamins, supplements, herbs etc [7]. While the patient should always consult with the physician before using cannabis along with any other prescription drugs, using CBD together with gabapentin may more efficiently help to decrease anxiety and stress, inflammation, and frequency and intensity of seizures while suppressing the adverse side effects of gabapentin alone [8]. Both, pregabalin and gabapentin have similar pharmacological mechanism of action, and both undergo elimination through renal system [9].
Gabapentin is the alkylated analog of GABA and is endorsed by the US FDA for the treatment of neuropathic pain and seizures. Gabapentin is suggested to act by impeding a particular alpha-2d subunit of voltage-gated calcium channel at specific presynaptic regions and, subsequently, to regulate the GABAergic mechanisms indirectly [10]. In view of the renal removal of these medications, no DDIs between the cannabinoids and gabapentinoidsought not expected. With reference to the efflux carriers, a few study outcomes proposed that a concomitant treatment of pregabalin with Pgp inhibitors allows the prolongation of its dose interval. Nevertheless, no investigations in the literature found higher levels of pregabalin in brain with the utilization of inhibitors of Pgp [11]. The potential pharmacokinetic interactions among CBD and antiseizure drugs (pregabalin and gabapentin) are mentioned in (Table 1) [12].
Table 1 : Antiseizure medication interactions with cannabidiol
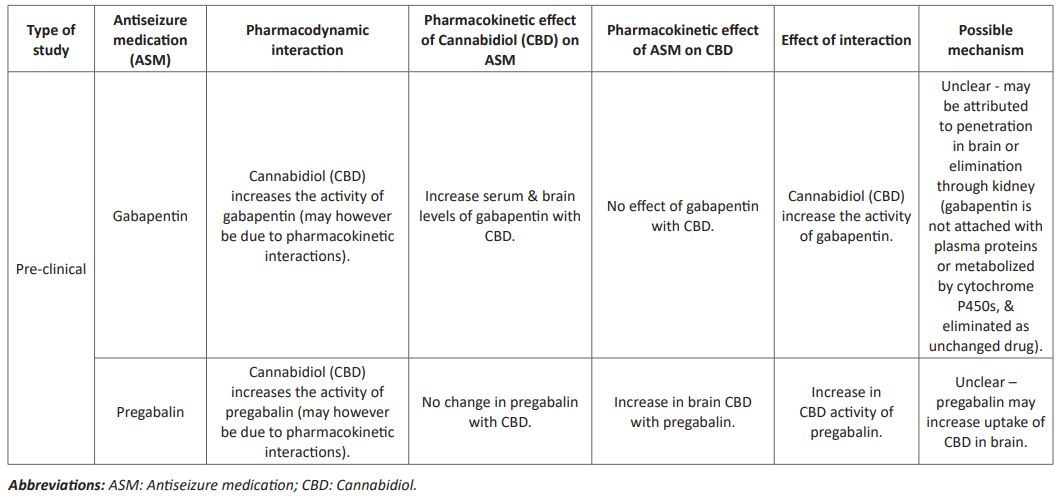
Cannabis are additionally purportedly abused when taken along with pregabalin and gabapentin. Gabapentin has lately come up as a promising option for the management of cannabis abuse among the adults [13,14]. Pre-clinical investigations propose that gabapentin regulates the corticotropin-releasing factor (CRF)-associated activation of GABA in the brain. This activation is related with the increase of in chances of alcohol dependence and, by generalization, to cannabis also, on the grounds that withdrawal from cannabis, similar to alcohol withdrawal, causes both anxiogenic-like state and greater release of extrahypothalamic CRF in the central part of amygdala in animals. The interaction of GABA and CRF and their part in the persuasive aspects of relapse of abuse give an compelling pre-clinical reasoning for the investigation of the efficacy of gabapentin in cannabis dependency. Besides, in clinical investigations of different ailments, gabapentin has been shown to lessen the craving and disturbances in mood and sleep, which are among the most relentless effects of extended cannabis withdrawal and the main cause the patients continue using cannabis. Gabapentin additionally showed a subtle cognitive improvement in the terms of concentration, attention, visual-motor activities, inhibition etc. in healthy individuals. Consequently, gabapentin, through calcium channel-GABAergic mechanistic action has importance for reestablishing the homeostasis in the normal brain stress system (CRF), may provide a novel management tactic comparative with agonistic, antagonistic, or psychiatric medications that have been investigated to date for the management of cannabis dependency 15].
In a study, 50 people looking for treatment for cannabis dependency were given 1200 mg/day of gabapentin in an 84-days double-blind, randomized, placebo controlled efficacy trial. Treatment with gabapentin reduced the cannabinoid (CB) metabolite levels in urine, self-reported score on use, cravings of cannabis, along with questionnaires about depression, and further enhanced the performance of executive function test, as compared to placebo controlled. Considering these positive results, and taking into account that there are no presently approved drugs for abuse of cannabis, investigation of the mechanistic pathways by which gabapentin act as a strong pharmacotherapeutic is expected to suggest future drug development endeavors.
The pharmacological action pathway for gabapentin has been basically associated to voltage-dependent calcium channels (VDCCs), particularly those containing the α2δ subunits. The VDCCs are present both in center and throughout periphery, and α2δ subunit is present across the various VDCC subtypes. Albeit the results of binding of ligand to the α2δ subunits have not been completely established, there seems to be an immediate effect on calcium conduction, as well as the VDCC transport, with the general results being a reduction in the neuronal action, which thus effects the movement of different chemical messenger. Additionally, the cannabinoid agonists affects the VDCC activities. One of the basic results of G-protein activation mediated by CB-receptor is the suppression of VDCCs. Moreover, there is proof for a CB-receptor independent regulation of VDCCs by the CB ligands, either via interaction with plasma membrane lipid bilayer or by the direct interface with the binding site on ion channel. Additionally, it is significant to note that the main role of the endogenous CBs is to regulate the release of different chemical messengers, which is a much described function of VDCCs.
One pre-clinical investigations seems to have explored the potential utilization of a VDCC ligand for the cannabis abuse. In this animal study, the effects of high efficacy CB agonist, CP-55,940 on the brain gene transcription, motor and anxiety were explored during the spontaneous withdrawal of CB. Pregabalin, which is considered as the future VDCC ligand because of its enhanced pharmacokinetic activity and higher bioavailability, diminished anatomic changes in the production of specific proteins which are considered to be involved in the development of cannabis dependency (i.e., the tyrosine hydroxylase enzyme in ventral tegmental region and the CB-1 receptors in nucleus accumbens) and weakened the motor and anxiety responses, induced by the cessation of cannabinoid agonist. Other investigations that has simultaneously assessed the gabapentinoids and cannabinoids involving the in vivo techniques is restricted and has comprised chiefly of the investigations involving the pre-clinical pain models. Those investigations have mostly showed that pregabalin, gabapentin, direct cannabinoid agonists and endogenous cannabinoid enzyme inhibitors act as analgesics under similar investigational pain conditions [16].
One of the clinical investigations compared nabilone (a non-particular CB agonist) with gabapentin in an open-label investigation in patients with neuropathic pain. Comparative with the baseline, results for sleep, pain, and anxiety were comparably improved by nabilone and gabapentin following the 3 and 6 months drug treatment. The cross study comparison also likewise showed the potential of VDCC ligands and CB agonists to further enhance the outcomes in terms of sleep, pain and anxiety. It is crucial to note is that difficulty in sleep, anxiety and physical distress are usually observed during cannabis restraint and are frequently reported as motive for continuous use. Taken together, these investigations showed a substantial therapeutic and neuropharmacological overlap between the cannabinoids and VDCC ligands that might play a role in the capacity of gabapentin and pregabalin in managing the addition of cannabis [17].
Conclusions
Constituents of cannabis interacts both at pharmacokinetic and pharmacodynamic levels with various antiseizure medications. The pharmacokinetic interactions have been reported between cannabis constituents and gabapentinoids. Not all these interactions, however, affect the therapeutic action, yet further larger clinical studies are warranted to determine how these drug interactions influence the clinical practice.
References
- https://www.healthline.com/health/what-is-cannabis#components
- Gilmartin CG, Dowd Z, Parker AP, Harijan P. Interaction of cannabidiol with other antiseizure medications: A narrative review. Seizure. 2021; 86: 189-96.
- Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012; 37(7): 1689-98.
- Vázquez M, Guevara N, Maldonado C, Guido PC, Schaiquevich P. Potential pharmacokinetic drug-drug interactions between cannabinoids and drugs used for chronic pain. BioMed research international. 2020.
- Alsherbiny MA, Li CG. Medicinal cannabis—potential drug interactions. Medicines. 2018; 6(1): 3.
- Finnerup NB, Jensen TS. Clinical use of pregabalin in the management of central neuropathic pain. Neuropsychiatric disease and treatment. 2007; 3(6): 885.
- https://www.drugs.com/drug-interactions/cannabis-with-gabapentin-2758-0-1147-0.html
- https://leafwell.com/blog/gabapentin-and-weed/
- Finnerup NB, Jensen TS. Clinical use of pregabalin in the management of central neuropathic pain. Neuropsychiatric disease and treatment. 2007; 3(6): 885.
- Weinstein AM, Miller H, Bluvstein I, Rapoport E, Schreiber S, et al. Treatment of cannabis dependence using escitalopram in combination with cognitive-behavior therapy: a double-blind placebo-controlled study. The American journal of drug and alcohol abuse. 2014; 40(1): 16-22.
- Elikottil J, Gupta P, Gupta K. The analgesic potential of cannabinoids. Journal of opioid management. 2009; 5(6): 341.
- Gilmartin CG, Dowd Z, Parker AP, Harijan P. Interaction of cannabidiol with other antiseizure medications: A narrative review. Seizure. 2021; 86: 189-96.
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern?. CNS drugs. 2014; 28(6): 491-6.
- Lile JA, Wesley MJ, Kelly TH, Hays LR. Separate and combined effects of gabapentin and Δ9-THC in humans discriminating Δ9-THC. Behavioural pharmacology. 2016; 27(2-3): 215.
- Brezing CA and Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018; 43(1): 173-194.
- Lile JA, Kelly TH, Hays LR. Separate and combined effects of the GABAB agonist baclofen and Δ9-THC in humans discriminating Δ9-THC. Drug and alcohol dependence. 2012; 126(1-2): 216-23.
- Lile JA, Wesley MJ, Kelly TH, Hays LR. Separate and combined effects of gabapentin and Δ9-THC in humans discriminating Δ9-THC. Behavioural pharmacology. 2016; 27(2-3): 215.

