Research Article - Volume 3 - Issue 1
Prevalence, Financial Losses and Risk Factors of Bovine Cysticercosis and Human Taeniasis in Selected Districts of Northwest Ethiopia
Nega Yismaw Alemu1; Abrham Ayele Tsegaye2*; Zewdu Seyoum Tarekegn2
1Centeral Gondar Zone Agricultural Office, Ethiopia.
2University of Gondar, College of Veterinary Medicine and Animal Sciences, Ethiopia.
Received Date : Nov 11, 2022
Accepted Date : Dec 17, 2022
Published Date: Jan 07, 2023
Copyright:© Abrham Ayele Tsegaye 2023
*Corresponding Author : : Abrham Ayele Tsegaye, University of Gondar,College of Veterinary Medicine and Animal Sciences, Ethiopia.
Email: ayeleabrham23@gmail.com
DOI: Doi.org/10.55920/2771-019X/1342
Abstract
Bovine cysticercosis, caused by the larval stage of the tapeworm, is a global zoonotic disease that affects cattle. Taenia saginata is the adult parasite that causes taeniasis in humans. To look into the prevalence and monetary costs of cysticercosis, a total of 384 cattle were recruited at three selected abattoirs; 180 questionnaires were distributed; and financial loss record sheets were seen. The overall prevalence of bovine cysticercosis was found to be 10.42%. 82.8% of the respondents encountered human taeniasis at least once in their lives. Bovine cysticercosis had resulted in ETB 294,217.33 losses from organ condemnation. About ETB 248, 983.90 expenses were recorded to buy 83,741 doses of taeniacidal drugs. In conclusion, bovine cysticercosis and human taeniasis were prevalent in the study areas and resulted in significant financial losses. Thus, integrated control systems should be adopted to control the burden.
Keywords: Bovine Cysticercosis, Human Taeniasis. Prevalence, Associated Risk Factors, Financial Significances, Northwest Ethiopia.
Introduction
Taeniasis is the intestinal infection of humans by the adult stages of cestode parasites under the genus Taenia. Taenia solium, Taenia saginata, and Taenia asiatica are the principal cestode specious important to cause taeniasis in human. Human acquires the adult stage and acts as a definitive host of the parasite through different risk factors. Outback human defecation and eating of raw or undercooked beef and/or pork are the primaries exposing factors [1]. Intermediate hosts of tapeworms could get cysticercosis, a disease caused by tissue infection of tapeworms, by ingestion of Taenia eggs. Cattle serve as an intermediate host for T.saginata and the cysticerci develop only in beef. T.solium and T. asiatica can preferably develop in pig visceral organs [2].
Bovine cysticercosis is caused by the larval stage of T.saginata. The eggs of those tapeworms (out or within the proglottids) are excreted with the faces of the infected final hosts to the external environment. Upon ingestion of these infective eggs, the intermediate hosts become infected and develop metacestode larval stages (also called cysticerci) in their muscles, resulting in bovine cysticercosis. People acquire taeniasis following ingestion of undercooked beef meat containing viable cysticerci [3].
Taeniasis and cysticercosis are highly prevalent in countries like Ethiopia, where poor hygienic conditions are coupled with poor cattle management practice and lack of strict meat inspection. National Hygiene and Sanitation Strategy Program reported that about 60% of the disease burden was related to poor hygiene and sanitation in Ethiopia [7]. The common habit of backyard slaughtering is also the key predisposing factor of taeniasis and bovine cysticercosis [4,5]. Around the globe 50 to77 million cases of taenia infection are recorded annually of which 50, 000 people are dying of it [6].
Cysticercosis causes significant economic impact in many parts of the world, particularly in developing countries especially by hindering the export of live animal and animal products. A high (50%) prevalence was recorded under large-scale management conditions [8] most probably because of direct exposure to proglottids shed from farm workers and sewage-contaminated feed or forage [9]. Hence, bovine cysticercosis is an important public health problem causing huge amount of economic losses [10]. Taeniasis is a well-known disease in Ethiopia with prevalence ranging from 10% to 70% [11]. However, information about bovine cysticercosis in Ethiopia is very limited. There is no study which is well documented to clearly show the status of taeniasis in humans and animals in parallel. The economic significances of Taenia infection and the number of losses due to Cysticercus bovis had not been well studied. Consequently, this study was conducted to give relevant information about the prevalence and financial losses of bovine cysticercosis and human taeniasis in the selected study areas; (Gondar, Bahir Dar, and Debre Tabor of northwest Ethiopia).
Materials/Methods
Study Areas
The study was conducted in Gondar, Debre Tabor, and Bahir Dar towns of Amhara National Regional State Ethiopia (Figure 1).
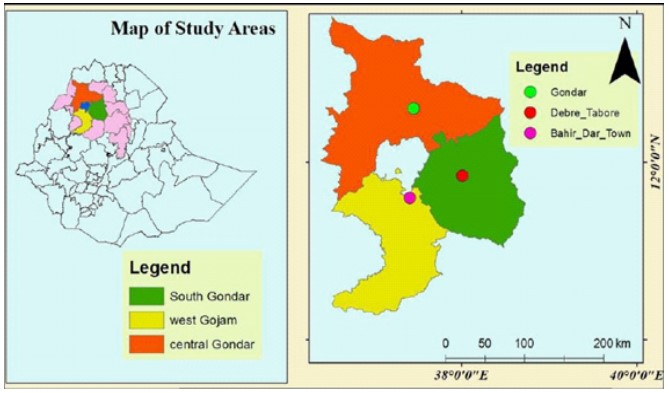
Figure 1: Locations of Study area (Gondar city, Debre Tabor ciry, and Bahir Dar city) (source: GIS data of Amhara Regional State Finance and Economics Bureau).
Study Design
A cross-sectional study was employed from November 2018 to May 2019 on cattle presented to Gondar, Debre Tabor, and Bahir Dar municipal abattoirs for slaughtering to estimate the prevalence of bovine cysticercosis; to see the distribution of these cysts among different organs of the animals and to characterize whether they were viable or degenerated. A retrospective study was employed to assess the trends of human taeniasis using record books from Gondar, Debre Tabor, and Bahir Dar referral hospitals. Prescriptions and drug sell documents in the respective hospitals’ drug shops were seen for the last 6 years (2014-2019) to assess costs lost for human taeniasis treatment. The taeniacidal drug cost supplied at each study hospitals’ shop was estimated using yearly adult taeniacidal drug doses. Documents in each studied municipal abattoirs were also visited to estimate the financial losses of C. bovis through organ condemnation. Losses associated with organ condemnations were made on abattoir records from 2014 to 2019. Moreover, a questionnaire survey was also conducted to assess the perception, experience, and knowledge of respondents about human taeniasis.
Sample Collection Technique
Before sampling, each selected anima was given an identification number at its initial get by writing a code with waterproof ink on its head. Starting from animals’ inlet of the abattoir; body condition, age, and breed of the animals were recorded on previously prepared data recording format. Temperature and feeding activity in the exercising place were intensively followed and recorded too. Animals brought for slaughter were categorized based on their age that 2-5 years as an adult and >5 years as old using their dentition as described by Ron et al. [15]. Body condition scoring was made based on the guideline provided by Klopcic et al., [16].
During the post–mortem inspection different predilection sites (liver, tongue, masseter muscle, heart, shoulder muscle, diaphragm, and intercostals muscle and thigh muscles) of the parasite were examined. Meat inspection was made according to Ethiopian Meat Inspection Regulations guideline and WHO meat hygiene keeping guideline in developing countries [17,18].
Sterile hypodermic needles were used to evacuate the pressure of cysts during the cyst fertility test. Cysticercus is characterized by its bladder-like transparent vesicle having two main parts: the vesicular wall and the scolex [19,20]. The presence of protoscolices in the cyst fluid was considered suggestive of fertility. Cysticerci of T. saginata were carefully removed using sterile scalpel blood and transported to laboratories near the abattoir. Cysts were then incubated at 37oc for 2 hours in a solution prepared from ox bile and normal saline at a proportion of 30:70. Size of cysticerci, presence of rostellum, and absence of hooks on the rostellum of the evaginated cyst were used to differentiate whether the cyst was T. saginata or another metacestode cyst [21]. Fertile cysts were subjected to a viability test. A drop of the sediment containing the protoscolices was placed on the microscopic slides and covered with cover slips and observed for amoeboid-like movements at 40X binocular microscope objective lens magnification. A drop of 0.1% aqueous eosin solution which was equal volume to the sediment containing protoscolices was added to create a clear vision. The technique differentiates the dead (red-stained) and alive (unstained) protoscoleces with the principle that viable protoscolices should completely or partially exclude the dye while the dead ones take it up [22].
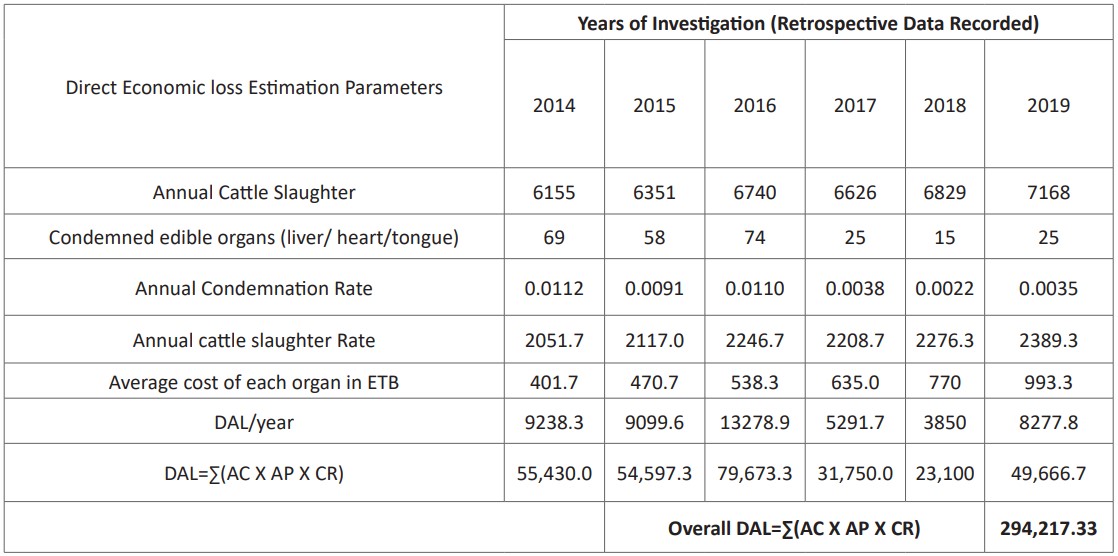
A questionnaire survey was conducted on 180 volunteer respondents. Data were also retrieved from hospitals’ record books and daily laboratory reports of stool examination of suspected patients. Similarly, an inventory of pharmaceutical shops of the studied hospitals was also considered to estimate the economic importance of taeniasis. Annual adult taeniacidal drug dose dispenses for patients and its worth was collected from record books to estimate human taeniasis associated with annual financial losses. Financial losses due to organ condemnation were computed using retrospective data obtained from the study abattoirs. Accordingly, the total direct financial loss was calculated by the formula given by Ogunrinade, A. and Ogunrinade, BI [23]: DAL=ΣAC×AP×CR; Where DAL = direct annual financial loss due to organs condemnation, AC = annual cattle slaughter rate of the abattoir, AP = average cost of each liver /heart/ tongue and CR = condemnation rates of liver/ heart/tongue).
Ethical Consideration
Ethical clearance was obtained from the Research and Ethical Committee of the University of Gondar, College of Veterinary Medicine and Animal Sciences, and Permission approval was also requested from each studied abattoir and hospitals. After getting permission, individual written consent was taken from each participant before administering the questionnaire.
Results
Cyst Prevalence, Distribution and Viability Test
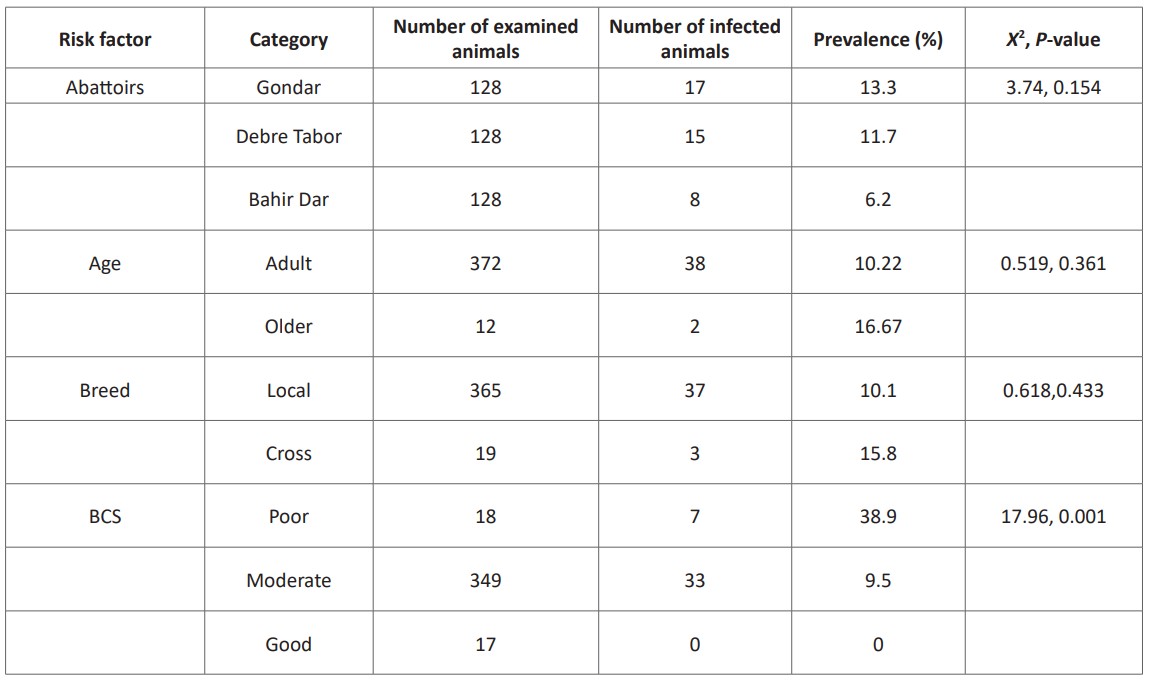
From the 384 cattle, 10.42% were infected with at least one cysticercus bovis in their organs or muscles. Abattoir wise prevalence of cysticercosis was 13.3% in Gondar, 11.7% in Debre Tabor, and 6.25% in Bahir Dar. In this survey, body condition score has shown a significant association (P < 0.05) with the prevalence of bovine cysticercosis (Table 1).
Table 1: Association of different risk factors with C. bovis infection in selected abattoirs.
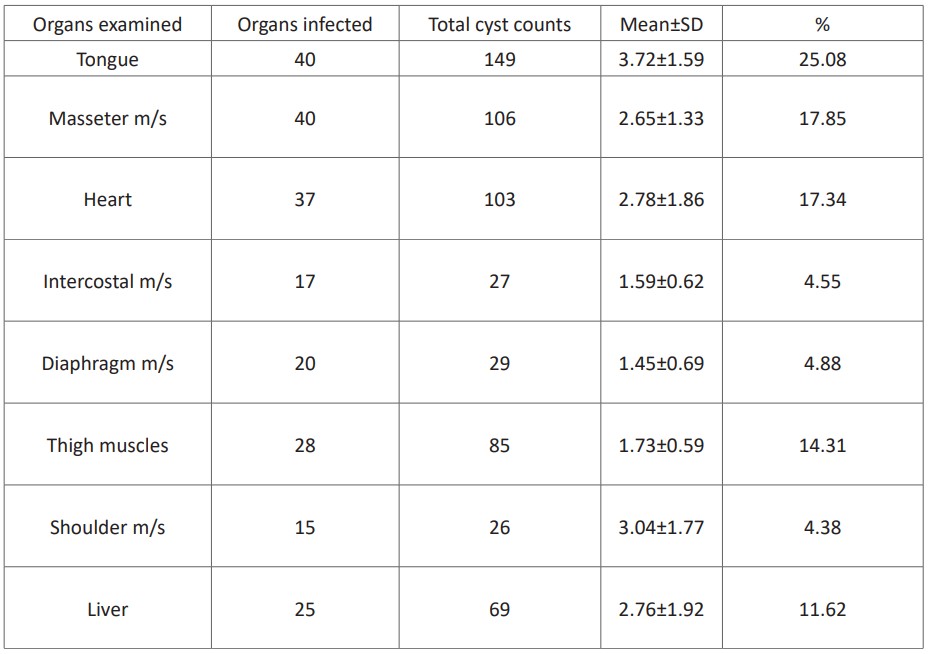
From 40 animals infected by C.bovis, 594 cysts were collected. The number of cysts recorded per organ or muscle ranged from 1 to 7. The tongue was constituted a higher number of cysts (25.08%) than the other organs /muscles. However, a relatively lower mean distribution of the cysts was recorded in the diaphragm (1.45±0.69). Out of the 594 cysts, 75.93% were found to be viable. The highest proportion of viable cysts (92.3%) was recorded from the shoulder muscle (Table 2).
Table 2: Mean±SD distribution of C. bovis cysts per infected organs.
Status of Human Taeniasis
82.8% of the respondents encountered T. saginata infection at least once in their life. The majority of the respondents (68.3%) had noted that they had an experience of raw and undercooked meat consumption and also confirmed that they knew taeniasis as an important meat-borne zoonotic disease. Only 52.7% of the participants have often accessed abattoirs service while others (47.2%) get meat from local butchers who supplied non inspected meat. 71% and 95% of the respondents who accessed meat from abattoir and local butchers respectively got T. saginata infection. Infected respondents have stated that they had experienced various symptoms including tapeworm segment expulsion (65.1%), abdominal discomfort (17.5%), abdominal bloating (14.8%), and not remember the signs they encountered (2.7%). Most infected participants (80%) have stated that taeniasis has resulted in treatment cost. Taenia infected respondents also confirmed that they use modern medicine (87%) and herbal medicine (12%) as their treatment options while others (1%) described that they had not treated the infection.
Financial Loss Estimations
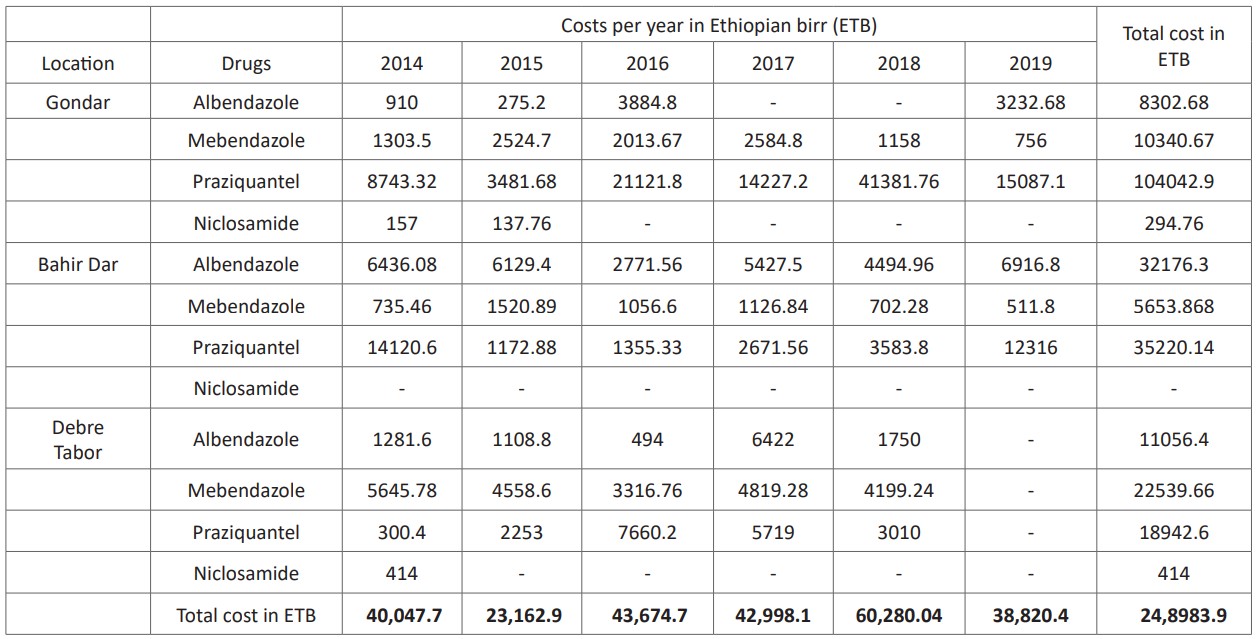
A total of 5,410 and 5,417 respective dosages of 200 and 400mg albendazole were used as human taeniasis treatment in the last six years in the study hospitals which worth ETB 51,535.38. Mebendazole shared a total of 23,866 dosages (for 100 mg) and 376 doses (for oral suspension), that worth about ETB 38,534.20. Praziquantel and niclosamide together had constituted to share a total dose of 48,672 doses with a worth of ETB 158, 205.64. In general, the inventory analysis showed that drug shops had sold about 83,365 doses and 376 suspensions in those areas for the last 6 years with worthies of ETB 248, 983.90 (Table 3).
Table 3: Annual taeniacidal drugs sold during 2014 to 2019 at 3 Hospitals pharmaceutical shops visited.
From 2014 to 2019 organ condemnation accounted about ETB 294,217.33 with the corresponding average organs cost of (liver ETB 47.5, heart ETB 57.33, and other main edible organs and muscles like thigh, and shoulder muscles ETB 186.67) (Table, 4).
Table 4: Cost of organ condemned (in ETB) from abattoirs during 2014 to2019.
Discussion
Bovine cysticercosis
In this study, the overall prevalence of bovine cysticercosis was found to be 10.42% (95% CI 7.6-13.8). It is comparable to the reports of Ashewani and Gebretsadik [24], Kebede et al. [25] and Regassa et al., [26] who reported an overall prevalence of 13.8%, 8.29%, 13.3% and 11.3%, respectively. However, a relatively lower prevalence of bovine cysticercosis had been reported in Ethiopia by Wondimagegn and Belete [27], and Tamerate et al. [28] with a prevalence of 4.64% and 4.2%, respectively. The variations between the present finding and the aforementioned reports might be associated with the performance of the meat inspector, sensitivity of the inspection procedures that can be affected by carcass type and incision method, abattoir facilities, the willingness of the owner, breed of the animal, environmental factors (climate), culture and socio-economic related activities of the communities (habit of raw meat consumption, personal and environmental hygiene).
Body condition score has a significant association with the prevalence of bovine cysticercosis. A higher infection rate was recorded in poor body condition scored cattle. This is in line with reports across the world [29]. C. bovis prevalence variation among different body condition scored animals might be related to the immune status of animals: animals with good body condition score can influence the establishment of cyst formation on muscles or organs by C. bovis.
C.bovis had been recorded in tongue, masseter muscle, heart, shoulder muscles, liver, diaphragm, intercostal muscle and thigh muscle with respective prevalence value of 10.42%, 10.42%, 9.4%, 7.3%, 6.51%, 4.95%, 4.17%, and 3.65%. It was in agreement with several previous reports [6,30,31,32]. Similar to Yacob et al. [34] and Tesfay and Assefa [35], the tongue and heart were the most commonly affected organs with bovine cysticercosis. A report from Iran [36] has shown that the most common preferred sites for the cysts were shoulder muscles followed by masseter muscles. Thigh, intercostals and diaphragm muscles were the least infected sites in this study. It was in agreement with the previous findings [32,33,37]. The activity of the muscles, the status of blood flow to the specific tissue, geographical location of the areas and the parasite features (strain) may determine the predilection sites of C. bovis in cattle [21,38].
Out of 594 cysts of C. bovis examined for viability test, 76% were viable while 24.07% have degenerated cysts. This report is higher as compared to previous reports by Abunna et al. [39] and Emiru et al. [40], who found 44.2% and 66.6% viable cysts respectably. The variations in the cyst viability in different localities might be associated with the difference in immunological response by infected individual cattle to the cyst and also the difference in cattle management activities by the producers [41]. The current study revealed that cysts from shoulder muscle were almost viable (92.31%) followed by the intercostal muscle (88.9%), diaphragm (86.2%), liver (84.1%) thigh muscles (82.4%), heart (68.9%), masseter muscle (69.8%), tongue muscle (70.47%). This is supported by a previous report by Harrison et al. [42]. This viability variation among predilection sites might be since the variation in metabolic reactions in each of the body organs.
Status of Human Taeniasis Using Questionnaire Survey
The 82.8% prevalence of taeniasis in a human was in line with different previous reports [33,34,37,43]. But it was relatively higher than the findings by Regassa et al. [26], Endris and Negussie [44], and Tegegne et al. [45]. This discrepancy might be associated with the level of community awareness about taeniasis, the differences of cysticercosis occurrence in cattle, the status of raw meat consumption, and the meat inspection procedures used. Similarly, studies have shown that the status of human taeniasis could be influenced by age, sex, religion, marital status, geographical location, educational status, and income of an individual [31,32,37,44]. This is true that in this survey religion, marital status, location, and household position of the respondents were declared as an important factor for the occurrence of human taeniasis.
The majority of the respondents (68.3%) in this assessment noted an experience of raw and undercooked meat consumption and also confirmed that taeniasis is an important public health problem in their areas. This supports the previous studies report that showed a strong association of T. saginata infection with the consumption of raw or undercooked beef [43,44]. The experience of human taeniasis was higher in Gondar (90%) followed by Debretabor (89.7%) and Bahirdar (69.4%). The variation across localities might be ascribed to the altitude that results in climate variation which influences the survival of the parasite in a contaminated environment, raw meat consumption habit, and hygienic status of the community.
Moreover, in this survey, beef and raw meat consumption, carcass source (from local butcher), and frequency of meat consumption had been recorded as the risk factor to influence the status of human taeniasis. This is supported by the reports of Terefe et al. [43] who stated that having uninspected meat from local butcher shops exposes the consumers more frequently than abattoir-originated meat. This author also declared that having raw or semi-cooked beef meat is a risk for public health concerns.
Retrospective assessment of human taeniasis
Out of 88,878 suspected patients, 903 (1.02%) were found to be infected by T. saginata in the last six years. In agreement with this report, comparable findings had been documented by Sah et al, (46) and Martinez-Maya et al., [47].
Economic loss estimations
A six years record from three hospitals’ pharmaceutical shops indicated that taeniacidal drugs worth 248,983.90 ETB for treatment purposes. The financial losses associated with bovine cysticercosis were accounted about 294,217.33 ETB with the corresponding average cost of organs (liver 47.5 ETB, heart 57.33 ETB, and other main edible organs and muscles like thigh, and shoulder muscles 186.67 ETB) based on the information given by the abattoir’s animal health control unit. In general, the direct economic losses due to the cost of the drug to human taeniasis and organ condemnation were estimated to be 543,201.23 ETB/ annum which equivalent to 18,731.07 USD. It was similar to a report by Tamirat et al. [28] and Van De [53].
Conclusion and Recommendations
10.42% of the total examined animals (384) were found to be positive for Cysticercus bovis cyst. The body condition of the slaughtered animals has a relationship with the prevalence of C. bovis that poor body condition of the animal was prevalent for of C. bovis infection. The anatomical distribution of Cysts was investigated and the result indicated that the proportion of viable cysts was higher than non-viable cysts. Among visceral organs examined; the tongue was with a high number of Cysticercus bovis. In interviewed respondents, the maximum infestation frequency by T. saginata was two times per year. Prevalence of taeniasis was associated with raw and undercooked beef consumption, presence of backyard slaughtering practices, open defecation, low level of public awareness and poor waste disposal. Household position; raw and semi-cooked meat consumption; religion and source of the carcass (local butchers and communal slaughtering) have a significant association with the occurrence of taeniasis. On the other hand, six years of retrospective data showed that 903/88,778 of those patients were stool positive for T. saginata egg. Inventories assessment indicated that 259,817 doses of taeniacidal drugs were used for medication with a worth of 543,201.23 ETB/ annum which equivalent to 18,731.07 USD.
Based on the above conclusion remarks, the following recommendations were forwarded:
- Sustainable community-based control strategies against zoonotic bovis(e.g. avoiding raw and undercooked meat consumption) should be designed and implemented.
- Strict measurements should be developed and practiced in abattoir meat inspection
- Back yard slaughtering should be controlled strictly.
- Experts and professionals in human medicine and veterinary science need to work in integration and collaboration about the causes and preventive mechanisms of bovis and human taeniasis.
- Further studies on the prevalence and economic importance of bovisand human taeniasis should be conducted in different zones of the regions
Acknowledgment
The author would like to acknowledge the University of Gondar in the financing of this study. University of Gondar veterinary Parasitology laboratory, Bahir Dar regional animal disease investigation and diagnostic center, and Debre Tabor veterinary clinic laboratory were also acknowledged for their universal logistics support and guide.
References
- Asaava LL, Kitala PM, Gathura PB, Nanyingi MO, Muchemi G. and Schelling, E. A Survey of Bovine Cysticercosis/Human Taeniosis In Northern Turkana District, Kenya. Preventive Veterinary Medicine. 2009; 89: 197-204.
- Murrell KD, Dorny P, Flisser A, Geerts S, Kyvsgaard NC, Mc Manus DP. WHO/FAO/OIE Guidelines for the surveillance, prevention, and control of taeniasis/cysticercosis. Geneva, Switzerland. WHO. 2005; 75-80.
- OIE. Terrestrial Manual-Cysticercosis. The version adopted by the World Assembly of Delegates: Chapter. 2013; 5: 452-456.
- Yanagida T, Sako Y, Nakao M, Nakaya K, Ito A. Taeniasis and cysticercosis due to Taenia solium in Japan. Parasit. Vectors. 2012; 5: 18.
- WHO. Foodborne disease outbreaks: guidelines for investigation and control. WHO Library cataloging in publication Data. 2004.
- Minozzo JC, Gusso RLF, Castro EAD, Lago O, Soccol VT. Experimental Bovine Infection with Taenia Saginata Eggs: Recovery Rates and Cysticerci Location. Brazilian Archives of Biology and Technology. 2002; 45: 451-455.
- FAO. Food and agriculture organization of the United Nations Rome. 2005.
- Eckert J. Workshop summary: of food safety, meat and fish borne Zoonoses. Veterinary Parasitology. 1996; 64: 143-147.
- Wayne L, N John, B Dave, S Brad. Outbreak of C bovis (T.saginata) in feedlot cattle in Alberta. Can. Vet. J. 2002; 43: 227-228.
- Wanzala W, JA Onyango-Abuje, EK Kang, KH Zessin, NM Kyule, et al. Control of Taenia saginata by post-mortem examination of carcasses. African Health Science. 2003b; 3: 68 76.
- Mamo E, Zein AZ, H Kool. Some common Zoonotic helminths In: The Ecology of Health, Ministry of Health, Addis Ababa. 1988; 231–243.
- CSA. Federal Democratic Republic of Ethiopia Central Statistical Agency Agricultural Sample Survey 2008/ 09 (2001 E.C) Report on Livestock and Livestock Characteristics. 2008; 2.
- Thrusfield M. Veterinary epidemiology. 2nd ed. UK: Blackwel Science. 2005; 178-87.
- Arsham H. Questionnaire design and Surveys sampling. 2002.
- Ron Torell, Ben Bruce, Bill Kvasnicka, Ken Conley. Methods of Determining Age of Cattle; Cattle Producer’s Handbook, fourth edition. 2003.
- Klopcic M, Hamoen A, Bewley J. Body Condition Scoring of Dairy Cows. Biotechnical Faculty, Department of Animal Science. 2011.
- Meat Inspection Regulations (No. 428); Federal Negarit Gazeta, 32nd Year, No. 14, 13th November. 1972.
- WHO. Report of round table conference on meat hygiene in developing countries. 1985; 4-8.
- Del Brutto OH. "Meningeal Cysticercosis". In Singh G, Prabhakar S. (eds.). Taenia solium cysticercosis from basic to clinical science. Wallingford, Oxon, UK: CABI Publishing. 2002; 177-188.
- Šlais Jaroslav, Serbus Ctibor, Schramlová Jana. "The microscopical anatomy of the bladder wall of Cysticercus bovis at the electron microscope level". Zeitschrift für Parasitenkunde. 1971; 36 (4): 304-320. [DOI:10.1007/BF00259638].
- Gracey G, Collins F and Huey R. Disease Caused by Helminthes and Arthropods Parasite Meat Hygiene.10th Ed. Uk; Saunders. 1993.
- Negash K, Beyene D, Kumsa B. Cystic echinococcosis in cattle slaughtered at Shashemanne Municipal Abattoir, South central Oromia, Ethiopia: Prevalence, cyst distribution and fertility. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2013; 107: 229-234.
- Ogunrinade A, Ogunrinade BI. Economic Importance of Bovine Fasciolosis in Nigeria. 1980.
- Ashwani K, Gebretsadik B. Occurrence of Cysticercosis in Cattle of Parts of Tigray Region of Ethiopia. Haryana Veterinarian. 2008; 47: 88-90.
- Kebede N, Tilahun G, Hailu A. Current Status of Bovine Cysticercosis of Slaughtered Cattle in Addis Ababa Abattoir, Ethiopia. Tropical Animal Health and Production. 2009; 41: 291-294.
- Regassa A, Abunna F, Mulugeta A, Megersa B. Major Metacestodes In Cattle Slaughtered at Wolaita Soddo Municipal Abattoir, Southern Ethiopia: Prevalence, Cyst Viability, Organ Distribution and Socioeconomic Implications. Tropical Animal Health and Production. 2009; 41: 1495.
- Wondimagegnei K, Belete, S. Prevalence and Public Health Significance of Cysticercus Bovis in and Around Debreberhan City. European Journal of Applied Sciences. 2015; 7: 199-208.
- Tamirat B, Tamirat H, Gebru M. Prevalence, Financial Impact and Public Health Significance of Cysticercus Bovis at Bahir Dar Municipal Abattoir, Ethiopia. Journal of Veterinary Medicine and Animal Health. 2017; 10: 1420.
- Kebede N. Cysticercosis of Slaughtered Cattle in Northwestern Ethiopia. Research in Veterinary Science. 2008; 85: 522 526.
- Megerssa B, Tesfaye E, Regassa A, Rahmeto A, Abunna F. Bovine Cysticercosis In Cattle Slaughtered at Jimma Municipal Abattoir, South Western Ethiopia: Prevalence, Cyst Viability, and Its Socio economic Importance. Vet. World. 2010; 3(6): 257-262.
- Bedu H, Tafess K, Shelima B, Woldeyohannes D, Amare B, Kassu, A. Bovine Cysticercosis in Cattle Slaughtered at Zeway Municipal Abattoir: Prevalence and It's Public Health Importance. Journal of Veterinary Science Technology. 2011; 2: 108.
- Dawit T, Tewodros S, Tilaye D. Public Health and Economic Significance of Bovine Cysticercosis in Wolaita Soddo, Southern Ethiopia. Global Veterinaria. 2012; 9: 557-563.
- Yacob H, Ahmed T, Getachew T, Tariku J. Bovine Cysticercosis and Human Taeniosis in Adama Town, Oromia Region, Ethiopia. Ethiopian Veterinary Journal. 2011; 15: 15-35.
- Tesfay H, Assefa A. Cysticercus bovis in Eastern Tigray, Northern Ethiopia. In. J. Innov. Sci. Res. 2012; 10: 2351-8014.
- Oryan A, Moghaddar N, Gaur Sn. Taenia Saginata Cysticercosis in Cattle with Reference to Its Prevalence, Pathogenesis and Economic Implications in Fars Province of Iran.Veterinary Parasitology. 1995; 57: 319-327.
- Abunna F, Tilahun G, Megersa B, Regassa A, Kumsa B. Bovine Cysticercosis in Cattle Slaughtered at Awassa Municipal Abattoir, Ethiopia: Prevalence, Cyst Viability, Distribution and It's Public Health Implication; zoonoses and Public Health. 2008; 5: 82-88.
- Belayneh G. Prevalence and Significance of Cysticercus bovis Among Slaughtered Cattle at Debre Zeit Abattoir. DVM Thesis. Faculty of Veterinary Medicine, Addis Ababa University. 1990.
- Abunna F. Prevalence, Organ Distribution, Viability and Socioeconomic Implication of Bovine Cysticercosis/Taeniasis, Ethiopia. Revue D’élevageet De Médecinevétérinaire des Pays Tropicaux. 2013; 66: 25-30.
- Emiru L, Tadesse D, Kifleyohannes T, Sori T, Hagos Y. Prevalence and Public Health Significance of Bovine Cysticercosis at Elfora Abattoir, Bishoftu, Ethiopia. Journal of Public Health & Epidemiology. 2015; 7: 34 40.
- Daryani A, Alaei R, Arab R, Sharif M, Dehghan M. The Prevalence, Intensity, and Viability of Hydatid Cysts in Slaughtered Animals in The Ardabil Province of Northwest Iran,” Journal of Helminthology. 2007; 81: 13-17.
- Harrison L, Sewell M, Macpherson C, Craig P. Parasitic Helminths and Zoonoses In Africa. 1991.
- Terefe Y, Redwan F, Zewdu E. Bovine cysticercosis and its food safety implications in Harari People’s National Regional State, eastern Ethiopia’, Onderstepoort Journal of Veterinary Research. 2014; 81(1): 676-682.
- Endris J, Negussie H. Bovine Cysticercosis: Prevalence, Cyst Viability and Distribution in Cattle Slaughtered at Kombolcha Elfora Meat Factory, Ethiopia. American Eurasian Journal of Agriculture and Environmental Science. 2011; 11: 173-176.
- Tegegne A, Hiko A, Elemo K. Bovine Cysticercosis and Human Taeniasis: Animal-Human Health and Economic Approach with Treatment Trends in Kombolcha Town, Wollo, Ethiopia. International Journal of One Health. 2018; 4: 15-21.
- Sah R, Pokharel P, Paudel I, Acharya A, Jha N, Bhattarai S. A Study of Prevalence of Taenia Infestation and Associated Risk Factors Among the School Children of Dharan. Kathmandu University Medical Journal. 2013; 10(3): 14-17.
- Martinez-Maya Jj, Aluja De, Avila-Ramirez As, Aguilar-Vega G, Plancarte- Crespo A, et al. Taeniosis and detection of antibodies against cysticerci among inhabitants of a rural community in Guerrero State, Mexico. 2013.
- Dawit S. Epidemiology of T. Saginata Taeniasis and Cysticercosis in North Gondar Zone. DVM Thesis, Faculty of Veterinary Medicine, Addis Ababa University. 2004.
- Hailu D. Prevalence and Risk Factor for Taenia Saginata Taeniosis / Cysticercosis in Three Selected Areas of Eastern Showa. MSc Thesis, Addis Ababa University, Faculty of Veterinary Medicine. 2004.
- Mulugeta A. Bovine Cysticercosis: Prevalence, Economic and Public Health Importance, DVM Thesis. Addis Ababa University, Faculty of Veterinary Medicine, Debre Zeit, Ethiopia. 1997.
- Eke S, Oguniyi T, Omalu I, Otuu C, Udeogu V, Luka J, et al. Prevalence of Human Taeniasis Among School Children in Some Selected Primary Schools In Bosso Local Government Area, Minna, Niger State, Nigeria. In. J. App. Bio Res. 2014; 6(2): 80-86.
- Rodriguez-R Canul, A Fraser, JC Allan, JL Dominguez-Alpizar, F Argaez-Rodriguez, et al. Epidemiological study of Taenia solium taeniasis/cysticercosis in a rural village in Yucatan state, Mexico, Annals of Tropical Medicine & Parasitology. 1999; 93(1): 57-67. [DOI: 10.1080/00034983.1999.11813395].
- Van De N, Le Th, Lien Pt, Eom Ks. Current Status of Taeniasis and Cysticercosis in Vietnam. Korean, Journal of Parasitology. 2014; 52: 12-59.

