Case Report - Volume 3 - Issue 1
A profound DTH-hypersensitivity reaction in a subject with suspected NSAID sensitivity given Celebrex
RM Gorczynski1*; CP Gorczynski2
1Institute of Medical Sciences, University of Toronto, Canada.
2Department of Immunology, Faculty of Medicine, University of Toronto, Toronto, ON, Canada.
Received Date : Nov 28, 2022
Accepted Date : Dec 19, 2022
Published Date: Jan 09, 2023
Copyright:© RM Gorczynski 2023
*Corresponding Author : : RM Gorczynski, Department of Immunology,Faculty of Medicine, University of Toronto, 429 Drewry Avenue, Toronto,M2R2K6, Canada.
Email: reg.gorczynski@utoronto.ca
DOI: Doi.org/10.55920/2771-019X/1343
Abstract
We report on a 49-year old male with a long history of pelvic/back pain (previously treated surgically) who was maintained for > 5 years on NSAIDs/gabapentin for pain relief. A marked dermatologic reaction developed at ~5 years of ongoing NSAID treatment, with hematuria and profound anemia (Hgb declined from 140 to 70m in 6 months) along with an intermittently pustular rash. OGD and colonoscopy did not suggest a significant GI bleed. After a brief (3 week) drug holiday a COX-2 inhibiting NSAID was prescribed, but within 3d a marked DTH reaction developed, consistent with a T cell cross reactivity between NSAIDs, ceasing within 2d of discontinuation of drug.
Introduction and clinical background
It is known that NSAIDs act though inhibition of cyclo-oxygenase (COX), the enzyme responsible for prostanoid biosynthesis, with COX-1 predominantly regulating protection in the GI and renal environment [1], while suppression of COX-2 is primarily responsible for the anti-inflammatory effects of NSAIDs [2]. Suppression of COX-1, with decreased PGE2 and increased leukotrienes is thought to cause the majority of both respiratory and dermatologic reactions to NSAIDs [3]. It has been implied that in the absence of an ASA-induced respiratory syndrome [3], COX-2 inhibitors may be used safely given the low risk of cutaneous reactivity [4,5].
More recently the understanding of adverse drug reactions (ADRs) to NSAIDs has been clarified by differentiating between high dose reactivity ( GI bleeding/tinnitus/renal disease)-type-”A” , and type-“B” ADRs which occur at doses harmless to most individuals [6]. Acknowledging that for Type-“B” reactions immediate reactivity is likely IgE mediated, and delayed reactivity is more likely to be T cell mediated, these authors then consider that it may be possible to use selective COX-2 inhibitory reagents in the latter individuals. However it has also been noted that an estimated 4% of individuals with a history of delayed rash to other NSAIDs are reported to develop a rash with celecoxib [7].
Case Observations
The hematuria and anemia seen in this patient are consistent with literature data describing ADRs (of type-“A” above [5]) to high doses of NSAIDs (this man admitted to using ~2gm naproxen daily), and both conditions abated on drug cessation. A dermatologist reviewing the rash diagnosed sebborheic dermatitis and adverse drug reactivity (possibly exanthematous pustulosis [8-10]. There was no concern regarding TEN or Stevens-Johnson syndrome [11]. Naproxen, which had been re-instituted at low dose for pain control, was stopped again, with topical steroid creams instituted. After ~3 weeks low dose (100mg) celebrex (selective COX-2 inhibitor) was initiated for ongoing pain relief. Within 2-3 days a severe hypersensitivity skin reaction developed, with marked erythema (see Figure1), which settled rapidly with drug cessation and first-generation anti- histamines used orally.
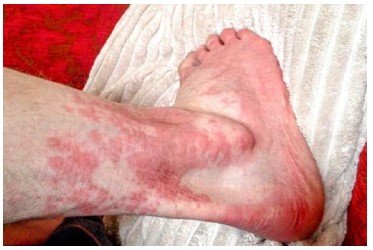
Figure 1: Macular erythematous rash on leg 48hr post initiation of Celebrex,100mg po, daily.
Conclusion
Despite confusing evidence in the literature regarding immunologic cross-reactivity between COX-1 and COX-2 inhibiting NSAIDs [3-6], there remains little evidence for type I (IgE mediated) hypersensitivity to this class of medications. However, the temporal and clinical observations seen in the subject described argue strongly for a likely T cell cross reactivity between COX-1 and COX-2 agents. We recommend extreme caution in use of COX-2 inhibitors in subjects who have any documented ADRsto non-selective NSAIDs use.
Conflict of interest statement: The authors confirm there are no conflicts of interest to disclose, financial or otherwise, in regards to this manuscript
References
- Munir MA, Enany N, Zhang JM. Nonopioid analgesics. Anesthesiol Clin. 2007; 25(4): 761-774.
- Gabay M, Tanzi M. Medications for chronic pain-nonopioid analgesics. Pract Pain Manage. 2011; 11(3): 92-101.
- Ferguson M, Piarowski L. Should patients with a history of NSAID sensitivity avoid all NSAIDs? NSAID Sensitivity. Pract Pain Manag. 2015; 15(7).
- Çelik GE, Erkekol FÖ, Aydın Ö, et al. Are drug provocation tests still necessary to test the safety of COX-2 inhibitors in patients with cross-reactive NSAID hypersensitivity? AllergolImmunopathol. 2013; 41(3): 181-188.
- Li L, Laidlaw T. Cross-reactivity and tolerability of celecoxib in adult patients with NSAID hypersensitivity. J Allergy Clin Immunol Pract. 2019; 7(8): 2891-2893
- Kowalski ML, Makowska JS. Seven steps to the diagnosis of NSAIDs hypersensitivity: how to apply a new classification in real practice? Allergy Asthma Immunol Res. 2015; 7(4): 312-20. [PMID: 25749768; PMCID: PMC4446629 DOI: 10.4168/aair.2015.7.4.312].
- https://www.aaaai.org/Allergist-Resources/Ask-the-Expert/Answers/2021/NSAID.
- Roujeau JC, Bioulac-Sage P, Bourseau C, Guillaume JC, Bernard P, Lok C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991; 127(9): 1333-8. [PMID: 1832534].
- Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) Br J Dermatol. 2007; 157: 989-996.
- Ward KE, Archambault R, Mersfelder TL. Severe adverse skin reactions to nonsteroidal antiinflammatory drugs: A review of the literature. Am J Health Syst Pharm. 2010; 67: 206-213.
- Mockenhaupt M, Kelly JP, Kaufman D, Stern RS SCAR Study Group. The risk of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with nonsteroidal antiinflammatory drugs: a multinational perspective. J Rheumatol. 2003; 30: 2234-2240.

