Case Report - Volume 3 - Issue 1
Complete remission of an unusual form of unspecified peripheral T-cell lymphoma: a case report and review of the literature
Nata Dieng1, El Hadji Daouda Niang2*, Ndeye Bougoul Seck3, El Hadji Souleymane Sarr4, Ndeye Marieme Diagne1
1Medical oncology and hematology Department, Principal Hospital, Dakar.
2Department of Clinical Hematology Dalal Jamm Hospital, Dakar, Senegal.
3Dermatology Department, Principal Hospital, Dakar.
4Pathological Anatomy and Cytology Department, Principal Hospital, Dakar
Received Date : Nov 17, 2022
Accepted Date : Dec 20, 2022
Published Date: Jan 10, 2023
Copyright:© El Hadji Daouda Niang 2023
*Corresponding Author : El Hadji Daouda Niang, Department of Clinical Hematology Dalal Jamm Hospital, Dakar, Senegal.
DOI: Doi.org/10.55920/2771-019X/1344
Abstract
Introduction: Peripheral T-cell lymphomas have a poor prognosis with less common sustainable remissions after standard anthracycline-based chemotherapy. We report a case of isolated skin involvement of an unspecified peripheral T-cell lymphoma with an unremarkable outcome under chemotherapy.
Observation: A 72-year-old hypertensive patient under treatment was seen in a hematology consultation for the management of a postero-lateral mass on theleft-hand first metacarpal, ulcerative-bulging with a clean background, rounded, firm, mobile, painless, measuring around 12 cm in length, and evolving for 4 months. Peripheral lymph nodes and other organs were unremarkable on examination. The patient underwent a biopsy of the mass.Histological examination of the specimen showed a malignant proliferation made up of medium-sized rounded cell layers with reduced cytoplasm and irregular nuclei with nucleated patterns sometimes. The stroma was densely fibrous and mitoses were quite frequent. On immunohistochemistry, the cells were CD3 positive, CD5, CD4, CCD8, CD7, CD25, CD20 and melan A negative. Perforin, FOXP3, CD30, and Pan cytokeratins were also negative. Ki67 was 80%. The hand X-raydidn’tidentify bone involvement. The biological work-up was unremarkable. The thoracic-abdominal-pelvic CT scan was normal. The diagnosis of nonspecific T-lymphoma stage IE Ann Arbor and IPI 2 was set. After 6 cycles of Cyclophosphamide-Doxorubicin-OncovinPrednisone chemotherapy, we obtainedcomplete tumor remission. MRI of the left hand aftermanagement showed a focal subcutaneous thickening opposite the 1st ray, without any underlying mass syndrome. After 3 months, the patient had a good general condition with no clinical recurrence.
Conclusion: This case illustrates an unusual location of an unspecified peripheral T-cell lymphoma with a complete response after standard anthracycline-based chemotherapy. The short-term prognosis is unremarkable.
Keywords: T-cell Lymphoma; Prognosis; Chemotherapy
Introduction
Peripheral T-cell lymphomas (PTCL) are a heterogeneous group of lymphoproliferative disorders originating from mature T cells [1]. They account for 15% to 20% of aggressive lymphomas and 5% to 10% of all non-Hodgkin’s lymphomas (NHL) [2,3]. One of the most common subtypes of PTCL is a heterogeneous group of nodal and extranodal mature T-cell lymphomas that do not fit into any of the specifically defined T-cell entities in the World Health Organization classification and are therefore termed PTCL, not otherwise specified (NOS) [4]. PTCL-NOS accounts for 26% of all PTCL [1]. Their prognosis is poor, most often involving nodal and extraganglionic sites, and overall survival (OS) is inferior to that of aggressive B-cell lymphomas [5]. We report a case of isolated skin involvement without ganglionic involvement of T-cell lymphoma that responded well to anthracycline-based chemotherapy
Observation
A 72-year-old hypertensive patient undergoing treatment was seen in a haematology consultation for the management of a postero-lateral mass on the left-hand first metacarpal, ulcerative-bulging with a clean background, rounded, firm, painless, measuring around 12 cm in length, and evolving for the past 4 months (Figure 1a). Peripheral lymph nodes and other systems were unremarkable. The patient underwent a biopsy of the mass. Histological examination of the specimen showed a malignant proliferation consisting of layers of medium-sized, rounded cells with reduced cytoplasm and irregular nuclei, sometimes nucleated. The stroma was densely fibrous and mitoses were quite frequent. On immunohistochemistry, the cells were CD3 positive, CD5, CD4, CCD8, CD7 and CD25 negative. CD20 and Melan A, perforin, FOXP3, CD30, and Pan cytokeratins were also negative. Ki67 was 80%. The blood count was unremarkable (Hb: 12.6 g/dl, Leukocytes: 5.3 G/l, and Platelets: 325 G/l). On Biochemistry, the LDH level was at 277 mg/l, serum protein electrophoresis was normal, and renal function was unremarkable (urea: 0.23g/l; creatinemia: 11mg/l). Serologies for hepatitis B, C, and HIV were negative. Hand X-ray showed no bone involvement. Cervico- horaco-abdominopelvic CT scan was normal. The diagnosis of unspecified T-cell lymphoma stage IE Ann Arbor and IPI 2 was made. After 6 cycles of Cyclophosphamide-Doxorubicin-Oncovin-Prednisone chemotherapy, complete tumor remission was obtained. MRI
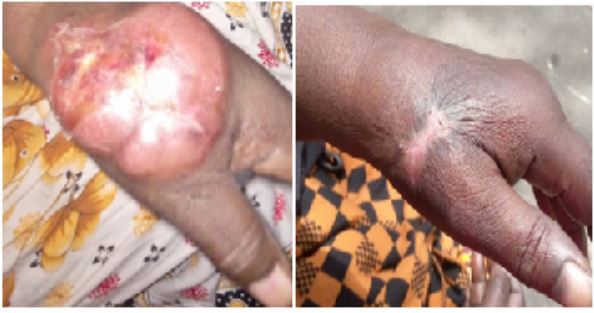
Figure 1: Evolution of the mass
a) Postero-lateral mass of the left hand opposite the first metacarpalbone, ulcerative-bulging, rounded, firm, mobile, painless, measuringaround 12 cm in length.
b) Complete remission of the mass with a scar opposite the biopsy site
of the left hand after treatment showed a focal subcutaneous thickening opposite the 1st ray, without any underlying mass syndrome. At 3 months follow-up, the patient is in good general condition with no clinical recurrence (Figure 1b).
Discussion
PTCL-NOS is the most common histological subtype (26%) of peripheral T-cell lymphomas (PTCL) [1]. It is a disease of the elderly, as previously reported, with a mean age at diagnosis of 60 years and a slight male predominance [6]. Compared with diffuse large cell B lymphomas, peripheral T lymphomas have a more extensive presentation and more extraganglionic involvement, particularly in the bone marrow and liver [6,7]. Skin involvement in peripheral T-cell lymphomas, whether tu moral or reactive (non-specific rash), is most commonly seen in angioimmunoblastic T-cell lymphomas [8]. However, they can be found in PTCL-NOS as an ulcerating mass described in our patient and other studies [9]. In its aggressive form, polyclonal hypergamma globulinemia is most often found in 14% [9] and autoimmunecytopeniain 3% of cases. In our patient, the early diagnosis would explain the absence of hematological and immunological disorders. On immunohistochemistry, PTCL-NOS is a diagnosis of exclusion characterized by an aberrant T-cell phenotype, with frequent loss of CD5 and CD7 and an absence of B-cell-associated antigens [10]. However, aberrant CD20 expression may be found [11]. Ann Arbor stage III/IV is most often noted in these patients at diagnosis, reflecting their aggressiveness [5,9], whereas the disease was located (stage IE) in our patient. However, 18F-fluorodeoxyglucose positron emission tomography (PET), unavailable in our setting, would be more effective in estimating the extent of the disease for staging and followup [6]. First-line treatment is based on anthracycline-based multidrug therapy with overall response rates of 50-60% and complete response rates of 20-30% [1,9]. However, no survival advantage was noted for patients with PTCL-NOS receiving anthracycline-containing combination chemotherapy compared with those receiving combination chemotherapy without anthracycline [9]. In our case, a complete response was achieved without clinical recurrence after 3 months (Figure 1b).
Conclusion
This case illustrates an unusual location of a non-specific peripheral T-cell lymphoma with a complete response after standard anthracycline-based chemotherapy. Its short-term prognosis is unremarkable. Close monitoring is necessary to outline early recurrence.
References
- Horwitz SM, Ansell S, Ai WZ, Barnes J, Barta SK, Brammer J,et al. T-CellLymphomas, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J NatlComprCancNetw. 2022; 20(3): 285‑308.
- Anderson JR, Armitage JO, Weisenburger DD, et al: Epidemiology of the non-Hodgkin’slymphomas: Distributions of the major subtypesdiffer by geographic locations. Ann Oncol. 1998; 717-720.
- Ascani S, Zinzani PL, Gherlinzoni F, et al: Peripheral T-celllymphomas: Clinico-patholigicstudy of 168 cases diagnosedaccording to the REAL classification. Ann Oncol. 1997; 8: 583-592.
- Swerdlow SH, Campo E, Harris TN L, et al. Classification OMS des tumeurs des tissus hématopoïétiques et lymphoïdes, Lyon, FranceAgence internationale pour la recherche sur le cance. 2008.
- Armitage JO. The aggressiveperipheral T-celllymphomas: 2015. Am J Hematol. 2015; 90(7): 665‑73.
- Oluwasanjo A, Kartan S, Johnson W, Alpdogan O, Gru A, Mishra A, et al. Peripheral T- ellLymphoma, not OtherwiseSpecified (PTCL-NOS). In: Querfeld C, Zain J, Rosen ST, éditeurs. T-Cell and NK-CellLymphomas [Internet]. Cham: Springer International Publishing. 2019; 176: 83‑98.
- Khan N, Clay C, Donati A. Peripheral T-celllymphoma, not otherwisespecified- Case report. Ann Med Surg. 2020; 56: 17‑8.
- Mourad N, Mounier N, Brière J, Raffoux E, Delmer A, Feller A, et al. Clinical, biologic, and pathologicfeaturesin 157 patients withangioimmunoblastic T-celllymphomatreatedwithin the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008; 111(9): 4463‑70.
- Weisenburger DD, Savage KJ, Harris NL, Gascoyne RD, Jaffe ES, MacLennan KA, et al. Peripheral T-celllymphoma, not otherwisespecified: a report of 340 cases from the International Peripheral T-cellLymphoma Project. Blood. 2011; 117(12): 3402-8.
- Savage KJ, Ferreri AJM, Zinzani PL, Pileri SA. Peripheral Tcelllymphoma – Not otherwisespecified. CritRevOncolHematol. 2011; 79(3): 321‑9.
- Horwitz SM, Ansell S, Ai WZ, Barnes J, Barta SK, Brammer J, et al. T-CellLymphomas, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J NatlComprCancNetw. 2022; 20(3): 285‑308.

