Case Report - Volume 3 - Issue 1
Platelet-rich fibrin used in regenerative endodontics and dentistry: Current uses, limitations, and future recommendations for application
Mamnoon Ghafir1; Nandita Gautam2*
1Department of Conservative Dentistry & Endodontics, Institute of Dental Sciences, Bareilly, India.
2Department of Public Health Dentistry, Institute of Dental Sciences, Bareilly, India.
Received Date : Nov 17, 2022
Accepted Date : Dec 24, 2022
Published Date: Jan 14, 2023
Copyright:© Nandita Gautam 2023
*Corresponding Author :Nandita Gautam, Department of Public Health Dentistry, Institute of Dental Sciences, Bareilly, India
Email: nanditagautam63552@gmail.com
DOI: Doi.org/10.55920/2771-019X/1348
Abstract
Numerous strategies, including pulp implantation, revascularization, and postnatal stem cell treatments, have been developed by regenerative endodontics. Revascularization has been effectively used in clinical settings recently, giving dentists amazing outcomes. When administered alone or in conjunction with a bone transplant, platelet-rich fibrin (PRF) encourages bone development and vascularization. There are many uses for PRF in regenerative endodontics, including as a tool for mending iatrogenic pulpal floor perforations and for revascularizing developing permanent teeth with necrotic pulps. It serves as a matrix for the ingrowth of tissue. It was seen that the dentinal walls thickened with time, the roots lengthened, the periapical pathology regressed, and the apex closed. The specific mechanism of action of PRF for dental pulp rejuvenation, including both vitro and in vivo, necessitates further study.
Keywords: Regenerative; Revascularization; Postnatal Stem Cell Therapy; PRF.
Introduction
Regenerative methods are those that include using substances to promote the healing and repair of the pulp-dentin complex after the infected or damaged tooth tissue has been restored [1]. Regenerative treatment in dentistry has the potential to revive a tooth that was previously considered to be dead. Various techniques, including pulp implantation, revascularization, and postnatal stem cell therapy, have been developed by regenerative endodontics. Revascularization has been effectively used in clinical settings recently, giving dentists astonishing outcomes [2]. Regenerative endodontics is crucial in resolving these issues because root canal therapy failures and postintervention problems are on the rise. The intention is to avoid radiography exposure and aggressive, intrusive instrumentation [3]. Reimposition of and T lymphocytes, which support defence against the microorganisms causing pulp injury, accomplishes this. The tooth's viability is maintained and the canal is entirely sealed, preventing periapical re - infection and teeth fractures [4].
Surgery is recognised to require healing, which is accomplished through a series of processes including cellular organisation, chemical signalling, and extracellular matrix for tissue repair [5]. When administered alone or in conjunction with a bone graft, platelet-rich fibrin encourages bone development and vascularization. The is matrix encourages osteoblast migration, cell adhesion, and proliferation, which results in the development of bone [6]. Blood vessel development and the stimulation of the immune system to combat foreign invaders are both significantly influenced by the cytokines generated by PRF [7]. According to research, low centrifugal force PRF preparation results in a similar effective concentration of leukocytes and growth factors as heavy centrifugal force preparation [8].
In patients with cleft alveolar ridge deformities, aresearch has demonstrated the improved outcomes of PRF consumption in combination with iliac crest bone transplant; in contrast, outcomes were unsatisfactory when iliac crest graft alone was employed [9]. Similar methods were used to complete healing without complications in an orthodontic surgery case employing PRF, cancellous bone allograft, bovine bone matrix, and metronidazole [10]. Thus according study findings, when PRF is mixed with biomaterials, the rejuvenation power of the corresponding alternative improves and it is more suited and acceptable for the defective tissue area. The appropriate inclusion of biomaterial is achieved thanks to PRF's development of cell-to-cell communication [11]. The current review's focus is on the uses of PRF in regenerative endodontics and dentistry, as well as its limits and possible future usage.
Materials and Composition
Platelet-rich fibrin (PRF), which is an artificial biomechanical structure, is made from homogenised plasma. This is accomplished by the fibrin clot that forms when human-derived blood is centrifuged. As a consequence of a polymerization reaction, this clot later comprises a large quantity of cytokines, growth factors, and platelets; aside from these components, no additional enzyme or anticoagulant is required for its formation. The cytokines in PRF that support osteoblast proliferation, angiogenesis, wound repair, and collagen synthesis are transforming growth factor (TGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), insulin growth factor-1 (IGF-1), fibroblast growth factor (FGF), and epidermal growth factor (EGF) [5], (Figure 1).
Classification of Plasma-Rich Fibrin
In transfusion medicine, platelet concentrates were originally used to stop haemorrhage caused by a variety of illnesses. Many decades ago, fibrin glues were made from blood-derived materials to seal wounds and hasten healing. Concentrated fibrinogen made up these fibrin glues [6]. Contamination risk is decreased by autologous origin [7]. Therefore, additional research in the area resulted in the substitution of platelet concentrates for fibrin glue, which was initially explained by Whitman et al. [8]. Consequently, platelet-rich fibrin (PRF) was first used in medicine as an aid for tissue regeneration in 2001 [9]. The idea was extrapolated from platelet-rich plasma, the first-generation platelet concentrate (PRP). Although the drawback of suppressing the coagulation cascade due of an anticoagulant agent, its effectiveness in numerous medical disciplines was astonishing with some more progression, Leukocyte PRF (L-PRF) came into being. It is labelled as such due to the higher leukocyte count. Because there are no of anticoagulants in the formulation, L-PRF functions as a three-dimensional fibrin matrix that traps growth factors [13-15]. Platelet concentrations were divided into four groups according to a suggested classification based on leukocyte and fibrin composition [16].
Leucocyte-Poor or Pure Platelet-Rich Plasma (P-PRP)
Pure platelet concentrates were initially created as a topical complement to traditional platelet units, and their first reported therapeutic application was in craniofacial surgeries [8,17]. With the help of a cell separator, platelets, leukocytes, and erythrocytes can be extracted from blood and utilised to create platelet concentrates for topical application. These elements can then be delivered to the patient again [18].
Leucocyte- and Platelet-Rich Plasma (L-PRP)
To implement platelet concentrates in routine practise without necessity for a transfusion center, a new, more practical technique was developed. At first, there were a lot of leucocytes in the product that made it difficult to remove them without a cell separator. However, the change in the collection criteria allowed for the acquisition of pure (leucocyte-free) PRP using more exacting testing. The primary drawback of this method is the demand for elaborate and pricey preparation kits and centrifuges.Additionally, the finished product dissolves fast, much like fibrin glue. As a result, L-PRP is no longer often used [19].
Leucocyte-Poor or Pure Platelet-Rich Fibrin (P-PRF)
Concentrates To produce pure platelet-rich fibrin, a smallquantity of blood is collected into a collection tube.
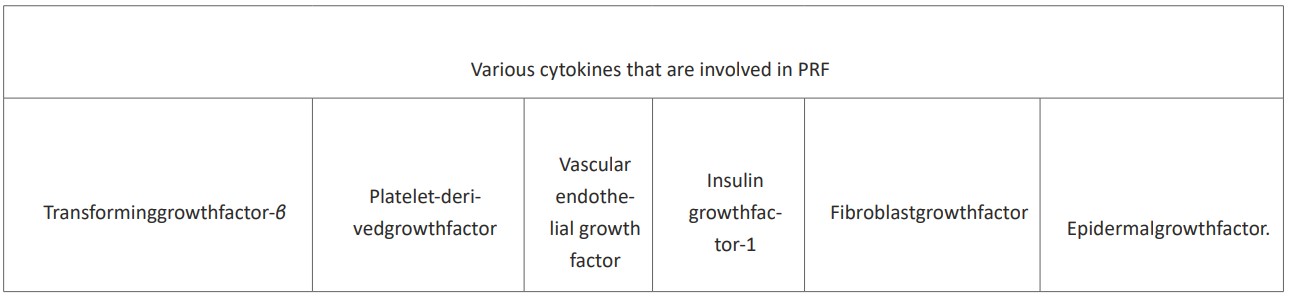
Figure 1: The Various cytokines that are involved in PRF.
Leucocyte-and Platelet-Rich Fibrin(L-PRF) Concentrates
In France, Choukroun et al. created a straightforward method for creating L-PRF [20]. Blood from the vena cava is taken into glass tubes and centrifuged slowly [21]. Due to the lack of anticoagulants, fibrin polymerization and platelet activation occur instantly. There are numerous clinical uses for the PRF clot in oral [22], maxillofacial [23,24], ENT [25], and plastic [26] surgery. Following administration, this preparation has the benefit of progressive breakdown, and the three-dimensional fibrin mesh gradually remodels, mimicking a physiological blood clot. The process is very easy to use and effective; larger quantities can be produced using just natural components as reagents. L-PRF is therefore most suited for routine procedures, and numerous nations, including France, Israel, and Italy, have indeed started using it [19].
Mechanism of Action of Platelet-Rich Fibrin
A compacted fibrin complex called PRF is made up of leucocytes, cytokines, and glycoproteins such thrombospondin. Leukocytes play a crucial role in the release of growth factors as well as an immunological response in a compacted PRF scaffold. This concentrated supply of platelets rich in growth factors aids in the promotion of tissue regeneration and wound healing. By promoting odontoblastic activity, transforming growth factor beta (TGF-) quickens reactive denti- nogenesis [27]. Infections and inflammatory pathways are inhibited by leukocytes, cytokines, and lymphocytes. Angiogenesis, a crucial step in the process of arterialization, is facilitated by vascular endothelial growth factor (VEGF) [28].
Role of Fibrin in Angiogenesis
The tri design of the fibrin matrix traps cytokines like FGF, VEGF, angiopoietin, and PDGF, causing a gradually increasing release that is essential for angiogenesis [29]. Endothelial cells are stimulated to bind to fibrin, fibronectin, and vitronectin when fibrin increases the expression of the v3 integrin [30].
Fibrin-Assisted Immune Response
Fibrin facilitates adhesion to endothelial cells, the production of fibrinogen, and neutrophil transmigration. On endothelial cells, fibrin works by increasing the expression of CD11c/CD18 receptors [31]. Fibrin and fibronectin control the macrophage colonization of wounds.
Effect of Fibrin on Mesenchymal Stem Cells
The fibrin matrix provides ascaffold for undifferentiated mesenchymal cells and promotes differentiation, imperative for tissuere generation [22].
Effect of Fibrin on Osseous Tissue
Parallel to how fibrin works as a scaffold for bone morphogenic protein, bone growth is induced by the prolonged release of this protein from the fibrin matrix. Angiogenesis is promoted by VEGF, FGF, and PDGF constant secretion. As a result of the circulating stem cells becoming trapped in the fibrin clot and causing hemostasis, tissue regeneration is made possible [32].
PRF in Dentistry
In surgical treatments like sinus lift surgeries, the use of PRF has been shown to have good success rates. Wound healing is also accomplished, and bone height and width are retained. In situations where immediate implants are to be inserted, PRF has been observed to significantly contribute to the extraction socket's quick healing. PRF also works synergistically with bone grafts in these situations. To get the clinical attachment loss, intrabony defects are treated with open flap debridement and PRF [33]. An avulsed tooth with a periapical abscess was treated by contouring the canal, and a triple antibiotic paste was applied until the follow-up, based on a case series. The antibiotic paste was then taken out in exchange, and the canal was watered.After revascularization, PRF was produced and placed in the canal, and then biodentine and glass ionomer cement were added. After six months of follow-up, PRF aided in the thickness of radicular dentin, apex closure, and apex regeneration [34].
Platelet-Rich Fibrin in Regenerative Endodontics
Endodontic treatment is necessary for a variety of disorders, including dental caries and pulpitis, which affect more than 2/3 of the worldwide people [35]. Furthermore, dental trauma in children causes pulpal tissue damage, which is especially concerning in developing teeth because there are few alternatives for therapy in cases of open apices [36]. The survival of the tooth has increased with the introduction of the revascularization therapy technique, nonetheless. It has had a positive effect on symptom relief, and postoperative radiograph show that the physiological root has been fully completed [37].
Revascularization has historically made it difficult to induce a blood clot in the root canal space, which has since been recognised as a therapeutic success [38,39]. Platelet concentrates were developed as an autologous scaffold for revascularization as a result [40]. In regenerative endodontics, platelet-rich fibrin (PRF) has many uses. When combined with MTA, it was utilised by Bains et al. to treat iatrogenic pulpal floor perforation of the mandibular first molar [41]. In order to revascularize immature permanent teeth with necrotic pulps, PRF is the best option since it offers a scaffold rich in growth factors that promotes cellular proliferation and differentiation. It serves as a matrix for the ingrowth of tissues [42]. Additionally, a continuous healing process is ensured by the growth factors' progressive release as the fibrin matrix resorbs [43]. According to Shivashankar et al., application of PRF on a teeth with pulpal necrosis and an exposed apex resulted in progressive thickness of the dentinal walls, root elongation, regression in the periapical lesion, and apical sealing [44].
Parallel to this, Rudagi K. and B. Rugadi reported effective healing and apexification with the combined use of MTA as an apical barrier and autologous platelet-rich fibrin membrane as an interior matrix [45]. Moreover, PRF boosted osteoprotegerin expression, alkaline phosphatase activity, and dental pulp cell proliferation in a time-dependent manner [46]. Positive results for pulpotomy in young permanent teeth using PRF have been reported [47]. Moreover, instead of solely using biomaterials for the bone augmentation following treatment of periapical defects, the combination of PRF with a bio- material (β-TCP) offers a better treatment alternative for swift healing [48]. It exhibits a more predictable radio- graphic and clinical bony regeneration [49].
Treatment with calcium hydroxide, which induces a calcific barriers but lessens the organic support of dentin (radicular), prevents root fracture in instances of traumatised young teeth with necrotic pulp. Platelet-rich fibrin is the greatest solution for preserving teeth life and strength in order to reduce the likelihood of fracture [42].
Figure 2: Role of PRF in healing of tissues.
Clinical Implementation
PRF in Oral and Maxillofacial Surgery (OMFS)
Investigators are now interested in the control of deformation in the alveolar bone that occur immediately after tooth extraction [50]. In comparison to spontaneous socket healing, the application of PRF resulted in less change in the alveolar bone's dimension prior to implant implantation, according to a study of 23 patients [51]. Additionally, it was shown that when the third molar extraction sockets were filled with PRF, there was an almost ten-fold decrease in the rate of osteomyelitis illnesses [52]. There are numerous additional applications for PRF in oral and maxillofacial surgery. In example, PRF is frequently used as a filling material in sinus lift and implant operations [53].The effectiveness of L-PRF in forming new bone and its function in wound healing have been demonstrated in numerous investigations. PRF-based membranes are used in treatments such alveolar ridge enhancement [54]. When similar membranes were utilised in patients on anticoagulation as well as the treatment and prevention of individuals with bisphosphonate-induced osteo- necrosis of the jaw, preliminary encouraging results were seen. After a tumour has been removed, L-PRF can be used to fill up the gaps [55] and ought to be further researched as a regenerative material. It can be utilised as an adjuvant with graft material (adipocytes) in plastic reconstructive procedures [26]. To take use of L-PRF features, additional research and comprehension of the right surgical technique are needed in other areas of OMFS, such as orthognathic surgery (Lefort osteotomies, etc.) [56].
PRF in Periodontics
PRF is used in the regeneration of the periodontium. It is enriched with soluble growth factors and cytokines, including TGF-β1, VEGF, ILGF, PDGF, and Il-1, -4, and -6, which mainly aid tissue regeneration and accelerate wound healing [57]. There is a notable improvement in the clinical outcome and the radiographic reduction in the intrabony defect depth when either bone grafts or pharmacological agents such as metformin gel is used in combination with PRF of being used alone [57]. Similarly, investigations of periodontal regeneration of class II furcation defects using PRF were studied. According to this, there was a significant improvement in clinical at- tachment loss (CAL) gains with the use of PRF when compared to open flap debridement (OFP). In conclusion, these results signify the tissue repair potential using PRF for furcation defects. Repair potential using PRF for furcation defects. In a research, the inclusion of PRF to the area after the lifting of a coronal advanced flap to cover gingival recession resulted in an early decrease in matrix metalloproteinase 8 and IL beta quantities and an increase in matrix metalloproteinase 1 level at day 10. As a consequence, the early stages of periodontal wound healing were promoted.
Future Recommendation
We need to apply platelet-rich fibrin during bone elevation treatments in avulsion and cystic excision cases where implants are inserted. Platelet-rich fibrin (PRF) is more affordable, simple to make, and practicable to employ in routine clinical procedures when contrasted to platelet-rich plasma (PRP). Platelet-rich fibrin (PRF) membranes are anticipated to be restored during periodontal treatments where guided tissue regeneration (GTR) is used to treat intrabony abnormalities. While platelet-rich plasma (PRP) cannot be used, they are suitable with people who have diabetes, smoke, or take anticoagulants. The fibrin membrane serves as a naturally occurring stimulating barrier, inhibiting the integration of soft and hard tissues, promoting bone regrowth, and filling the defect. The specific mechanism of PRF's activity for dental pulp regeneration, both in vitro and in vivo, has to be clarified through research.
References
- PE Murray, F Garcia-Godoy, KM Hargreaves. Regenerative endodontics: a review of current status and acallforaction, Journal of Endodontics. 2007; 33(4): 377-390.
- D Johns, V Shivashankar, S Krishnamma, M Johns. Use of photoactivated disinfection and platelet-rich fibrin in regenerative endodontics, Journal of Conservative Dentistry. 2014; 17(5): 487.
- N Shah, A Logani, U Bhaskar, V Aggarwal. Efficacy ofrevascularization to induce apexification/apexogensis in in-fected,nonvital, immatureteeth: apilotclinicalstudy, Journal of Endodontics. 2008; 34(8): 919-925.
- CM Sedgley, HH Messer. Are endodontically treated teeth more brittle?, Journal of Endodontics. 1992; 18(7): 332-335.
- SV Khiste, R NaikTari. Platelet-rich fibrin asabiofuel for tissuere generation, International Scholarly Research Notices. 2013; 6. ArticleID627367.
- H Matras. Die wirkungen vershiedener fibrin praparate aufkontinuitat-strennungen der rattenhaut, Osterr Z Stomatol. 1970; 67(9): 338-359.
- J Gibble and P Ness. Fibrin glue: the perfect operative sealant, Transfusion. 1990; 30(8): 741-747.
- DH Whitman, RL Berry, DM Green. Platelet gel: anautologous alternative to fibrin glue with applications in oral and maxillofacial surgery, Journal of Oral and Maxillofacial Surgery. 1997; 55(11): 1294-1299.
- J Choukroun, F Adda, C Schoeffler, A Vervelle. Opportunities in implant dentistry: PRF, Implantodontie. 2001; 42(62).
- G.Anfossi, M. Trovati, E Mularoni, P Massucco, G Calcamuggi, G Emanuelli. nfluence of propranololon platelet aggregation and thromboxane B2 production fromplatelet-rich plasma and whole blood, Prostaglandins, Leu-kotrienes and Essential Fatty Acids. 1989; 36(1): 1-7.
- R Fijnheer, R Pietersz, D Korte, et al. Platelet activation during preparation of platelet concentrates: a comparison of the platelet-rich plasma and the buffy coat methods,Transfusion. 1990; 30(7): 634-638.
- RE Marx. Platelet-rich plasma: evidence to support its use, Journal of Oral and Maxillofacial Surgery. 2004; 62(4): 489-496.
- M Toffler. Guided bone regeneration (GBR) using corticalbone pins in combination with leukocyte- and platelet-richfibrin (L-PRF), Compendium of Continuing Education in Dentistry. 2014; 35(3): 192-198.
- V Lekovic, I Milinkovic, Z Aleksic, et al. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects, Journal of Periodontal Research,. 2012; 47(4): 409-417.
- VY Shivashankar, DA Johns, S Vidyanath, and G Sam. Combination of platelet rich fibrin, hydroxyapatite and PRF membrane in the management of large inflammatory peri-apical lesion, Journal of Conservative Dentistry: JCD. 2013; 16(3): 261-4.
- M Del Corso, Z Mazor, JL Rutkowski, DM D Ehrenfest. The use of leukocyte- and platelet-rich fibrin during immediate post extractive implantation and loading for the esthetic replacement of a fractured maxillarycentral incisor, Journal of Oral Implantology. 2012; 38(2): 181-187.
- RE Marx, ER Carlson, RM Eichstaedt, SR Schimmele, JE Strauss, et al. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 1998; 85(6): 638-646.
- G Weibrich, WKG Kleis, G Hafner, WE Hitzler, W Wagner. Comparison of platelet, leukocyte, and growthfactor levels in point-of-care platelet-enriched plasma, pre-pared using a modified curasankit,with preparations received from a local blood bank, Clinical Oral Implants Research. 2003; 14(3): 357-362.
- DM Dohan Ehrenfest, L Rasmusson, T Albrektsson. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) toleucocyte-andplatelet-rich fibrin (L-PRF), Trends in Biotechnology. 2009; 27(3): 158-167.
- B Naik, P Karunakar, M Jayadev, VR Marshal. Role of platelet rich fibrin in wound healing: a critical review, Journal of conservative dentistry: JCD. 2013; 16(4): 284-293.
- DM Dohan, M DelCorso, JB Charrier. Cytotoxicity analyses of choukroun’s platelet-rich fibrin (PRF) on a wide range of human cells: The answer to a commercial controversy, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2007; 5(103): 587-593.
- J Choukroun, A Diss, A Simonpieri, et al. Platelet-richfibrin (PRF): a second-generation platelet concentrate. partIV: clinical effects on tissue healing, Oral Surgery, OralMedicine, Oral Pathology, Oral Radiology, and Endodontology. 2006; 101(3): 56-e60.
- J Choukroun, A Diss, A Simonpieri, et al. Platelet-rich fibrin(PRF): A second-generation platelet concentrate. partV: histologic evaluations of PRF effects on bone allograft mat-uration in sinus lift, Oral Surgery, Oral Medicine, Oral Pa-thology, Oral Radiology, and Endodontology. 2006; 101(3): 299-303.
- A Diss, DM Dohan, J Mouhyi, P Mahler. Osteotomesinus floor elevation using choukroun’s platelet-rich fibrin asgrafting material: a1-year prospective pilot study with micro threaded implants, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2008; 105(5): 572-579.
- JI Choukroun, F Braccini, A Diss, G Giordano, P Doglioli, DM Dohan. Influence of platelet rich fibrin (PRF) onproliferation of human preadipocytes and tympanic kerati-nocytes: a new opportunity in facial lipostructure (Coleman'stechnique) and tympanoplasty?, Revue de laryngologie-oto-logie-rhinologie. 2007; 128(1-2): 27-32.
- F Braccini and D Dohan. The relevance of choukroun’splatelet rich fibrin (PRF) during facial aesthetic lipostructure (coleman’s technique): preliminary results, Revue de Lar-yngologie Otologie Rhinologie. 2007; 128(4): 255-260.
- AJ Smith, PE Murray, AJ Sloan, JB Matthews, S Zhao. Trans-dentinal stimulation of tertiary dentinogenesis, Advances in Dental Research. 2001; 15(1): 51-54.
- M Toffler, D Holtzclaw, M del Corso, and N Toscano. Introducing choukroun’s platelet rich fibrin (PRF) to there constructive surgery milieu, Journal of Implant and Ad-vanced Clinical Dentistry. 2009; 1(6): 21-30.
- HF Dvorak, VS Harvey, P Estrella, LF Brown, J McDonagh, AM Dvorak. Fibrin containing gelsinduce angiogenesis. Implications for tumor stroma genera-tion and wound healing,”Laboratory Investigation; A Journal of Technical Methods and Pathology. 1987; 57(6): 673-686.
- X Feng, RAF Clark, D Galanakis, MG Tonnesen. Fibrin and collagen differentiallyregulate humandermalmicrovascularendothelialcellintegrins:stabilizationofαv/β3mRNA by fibrin1, Journal of Investigative Dermatology. 1999; 113(6): 913-919.
- JD Loike, B Sodeik, L Cao, et al. CD11c/CD18 on neu-trophils recognizesa domain at the N terminus of the A alphachain of fibrinogen, Proceedings of the National Academy of Sciences. 1991; 88(3): 1044-1048.
- M Kawamura, MR Urist. Human fibrin isaphysiologic delivery system for bone morphogenetic protein, Clinical Orthopaedics and Related Research. 1988; 235: 302-310.
- RJ Miron, G Zucchelli, MA Pikosetal. Use of platelet-rich fibrin in regenerative dentistry: a systematic review, Clinical Oral Investigations. 2017; 21(6): 1913-1927.
- H Bakhtiar, S Esmaeili, S Fakhr Tabatabayi, MR Ellini, MH Nekoofar, PMH Dummer. Second-generationplatelet concentrate (platelet-rich fibrin) as a scaffold in re-generative endodontics: a case series, Journal of Endodontics. 2017; 43(3): 401-408.
- T Larsen, NE Fiehn. Dental biofilm infections-anupdate,Apmis. 2017; 125(4): 376-384.
- HL Ray, J Marcelino, R Braga, R Horwat, M Lisien, S Khaliq. Long-term follow up of revascularization using platelet-rich fibrin, Dental Traumatology. 2016; 32(1): 80-84.
- T Jeeruphan, J Jantarat, K Yanpiset, L Suwannapan, P Khewsawai, KM Hargreaves. Mahidol study: comparison of radiographic and survival outcomes of im-mature teeth treated with either regenerative endodontic ora pexification methods: A retrospective study, Journal of Endodontics. 2012; 38(10): 1330-1336.
- RY Ding, GSP Cheung, J Chen, XZ Yin, QQ Wang, CF Zhang. Pulp revascularization of immature teeth with apical periodontitis: A clinical study, Journal of End-odontics. 2009; 35(5): 745-749.
- ZC Cehreli, B. Isbitiren, S. Sara, and G. Erbas, Regenerative endodontic treatment (revascularization) of immaturene-crotic molars medicated with calcium hydroxide: A case se-ries, Journal of Endodontics. 2011; 37(9): 1327-1330.
- A Nosrat, A Seifi, S Asgary. Regenerative endodontictreatment (revascularization) for necrotic immature perma-nent molars: a review and report of two cases with a new biomaterial, Journal of Endodontics. 2011; 37(4): 562-567.
- R Bains, VK Bains, K Loomba, K Verma, A Nasir. Management of pulpal floor perforation and grade II Fur-cation involvement using mineral trioxide aggregate and platelet rich fibrin: A clinical report, Contemporary Clinical Dentistry. 2012; 3(2): S223-S227.
- D Keswani, RK Pandey. Revascularization of an im-mature tooth with a necrotic pulp using platelet-rich fibrin: acase report, International Endodontic Journal. 2013; 46(11): 1096-1104.
- A Simonpieri, M Del Corso, G Sammartino, DM Dohan Ehrenfest. There levanceofchoukroun’s platelet-rich fibrin and metronidazole during complex max-illary rehabilitations using bone allograft partI: A new grafting protocol, Implant Dentistry. 2009; 18(2): 102-111.
- VY Shivashankar, DA Johns, S Vidyanath, MR Kumar. Platelet rich fibrin in the revitalization of tooth with necrotic pulp and open apex, Journal of Conservative Dentistry: JCD. 2012; 15(4): 395-8.
- K Rudagi and B Rudagi. One-step apexification in imma-ture tooth using grey mineral trioxide aggregate as an apicalbarrier and autologus platelet rich fibrin membrane as aninternal matrix, Journal of Conservative Dentistry. 2012; 15(2): 196.
- FM Huang, SF Yang, JH Zhao, and YC Chang. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells, Journal of Endodontics. 2010; 36(10): 1628-1632.
- H Hiremath, S Saikalyan, SS Kulkarni, V Hiremath. Second-generation platelet concentrate (PRF) as a pulpot-omy medicament in a permanent molar with pulpitis: a casereport, International Endodontic Journal. 2012; 45(1): 105-112.
- BJ Kim, TK Kwon, HS Baek, et al. A comparative studyof the effectiveness of sinus bone grafting with recombinant human bone morphogenetic protein 2-coated tricalcium phosphate and platelet-rich fibrin-mixed tricalcium phosphate in rabbits, Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2012; 113(5): 583-592.
- K Jayalakshmi, S Agarwal, MP Singh, BT Vishwanath, A Krishna, R Agarwal. Platelet-rich fibrinwith β-tri-calcium phosphate-a noval approachfor bone augmentationin chronic periapical lesion: a case report, Case Reports in Dentistry. 2012; 6. Article ID: 902858.
- V DeRisi, M Clementini, G Vittorini, A Mannocci, M DeSanctis. Alveolar ridge preservation techniques: asystematic review and meta-analysis of histological and his-to morphometrical data, Clinical Oral Implants Research. 2015; 26(1): 50-68.
- F Hauser, N Gaydarov, I Badoud, L Vazquez, JP Bernard, P Ammann. Clinical and histological evaluation of post extraction platelet-rich fibrin socket filling, Implant Dentistry. 2013; 22(3): 295-303.
- DR Hoaglin and GK Lines. Prevention of localized osteitis in mandibular third-molarsites using platelet-richfibrin, International Journal of Dentistry. 2013; 4. Article ID: 875380.
- M DelCorso, M Toffler, D Dohan Ehrenfest. Use of anautologous leukocyte and platelet-rich fibrin (L-PRF) mem-braneinpost-avulsionsites: an over view of choukroun’sPRF, Journal of Implant and Advanced Clinical Dentistry. 2010; 1(9): 27-35.
- M Tatullo, M Marrelli, M Cassettaetal. Platelet rich fibrin (PRF) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations, International Journal of Medical Sciences. 2012; 9(10): 872-880.
- JB Charrier, JP Monteil, S Albert, S Collon, S Bobin, DM Dohan Ehrenfest. Relevance of choukroun’s platelet-rich fibrin (PRF) and SMAS flap in primary reconstruction aftersuperficial or subtotal parotidectomy in patients with focal pleio morphic adenoma: A new technique, Revue de Laryngologie Otologie Rhinologie. 2008; 129(4-5): 313-318.
- A Simonpieri. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: bone graft, implant and reconstructive surgery, Current Pharmaceutical Biotechnology. 2012; 13(7): 1231-1256.
- UP Verma, RK Yadav, M Dixit, A Gupta. Platelet-rich fibrin: a paradigm in periodontal therapy–a systematicreview, Journal of International Society of Preventive & Community Dentistry. 2017; 7(5): 227.

