Case Report - Volume 3 - Issue 1
Recurrent thrombosis in a lady: Is there a missed connection?
Deepanjan Bhattacharya1*; Koyel Chakraborty2; Rohit Walse1; Harikrishnan Kurup KN1; Bijulal Sasidharan1; Ajit kumar1
1Department of Cardiology, Sree Chitra TirunalInsitute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, India.
2Regional Institute of Ophthalomology, Medical College Kolkata, India.
Received Date : Nov 17, 2022
Accepted Date : Dec 28, 2022
Published Date: Jan 18, 2023
Copyright:© Berrada Kenza 2023
*Corresponding Author : Deepanjan Bhattacharya, Senior Resident, Department of Cardiology, Sree Chitra TirunalInsitute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, India.
Email: b.deepanjan@yahoo.co.in
DOI: Doi.org/10.55920/2771-019X/1351
Abstract
Background: COVID19 infection is a recognized risk factor for systemic thrombosis. There have been few case reports about thrombosis following COVID19 vaccination.
Case presentation: A middle aged lady presented with acute pulmonary thromboembolism, with history of cerebral sinovenous thrombosis 3 weeks back. Thrombophilia workup and DVT screen was negative, but on reviewing history she had received CoviShield vaccine 1 week prior to the CSVT.
Conclusion: COVID19 vaccine use is an under-reported cause for systemic thrombosis. However, larger studies are required to establish the mechanism and causal-effect relationship.
Keywords: Left superior vena cava; congenital.
Case Presentation
A 46-year-old lady, known case of hypertension, dyslipidemia and hypothyroidism, presented with history of progressive dyspnea on exertion and chest discomfort at exertion which progressed to rest for past 4 days. She had a recent admission 3 weeks prior to presentation, during which CT showed infarct in right insula and capsuloganglionic region, and MR venogram confirmed cerebral sinovenous thrombosis (CSVT) (Figure 1). Thrombophilia workup includinganti-nuclear antibody, anticytoplasmic antibodies and antiphospholipid antibody workup were negative. Patient was started on oral rivaroxaban at 15 mg daily dosage following the event. Incidentally, she had received 1st dose of COVID19 vaccine (CoviShield) 1 week prior to the CSVT. There was no history of prior thrombosis, recurrent abortions or oral contraceptive pill use.
On examination, she was tachypneic, had tachycardia (HR 112/min) with normal blood pressure (110/60 mm Hg). Jugular venous pressure was not raised and there was no pallor or pedal edema. Cardiovascular examination revealed normal 1st and 2nd heart sounds with right ventricular third heart sound and no murmurs. Systemic examination was otherwise unremarkable.
Electrocardiogram showed sinus tachycardia with T wave inversion in III (Figure 2). Echocardiogram demonstrated good LV systolic function with dilated right atrium and right ventricle with right ventricular dysfunction. Chest X ray showed left sided pleural effusion with normal cardiac silhouette. Suspecting pulmonary thromboembolism, a CT pulmonary angiogram was ordered which showed thrombus in the hilum of right pulmonary artery (Figure 3). NT pro BNP was elevated (1870 ng/ml). Lower limb Doppler did not show any evidence of deep venous thrombosis. Her renal and liver function tests were unremarkable. An RTPCR for SARS COVID 19 virus was negative. Covid antibodies (IgM and IgG) were also negative.
In view of RV dysfunction with high risk PE (PESI score 106, class IV), it was decided for systemic thrombolysis with tenecteplase, which was performed uneventfully. She was treated with mg of tenecteplaseand continued on heparin infusion. A repeat CTPA did not show any evidence of resolution of thrombus, and there was persistence of RV dysfunction. Patient continued to be sick requiring ionotropic support and was planned for mechanical thrombectomy as a bailout strategy. However she developed refractory hypotension and bradycardia prior to procedure and could not be revived.
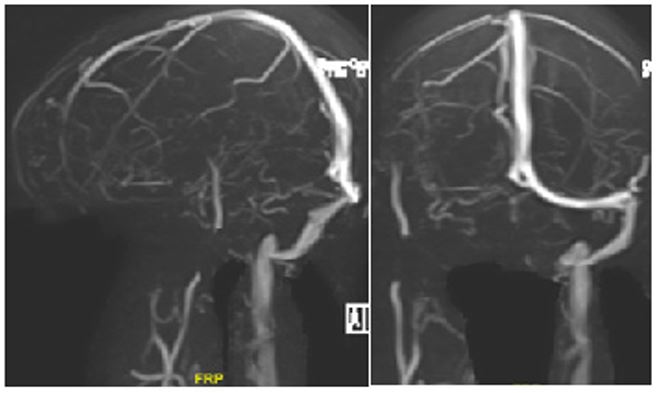
Figure 1: MR venogram showing non-visualisation of right transverse sinus, sigmoid sinus and right internal jugular vein.
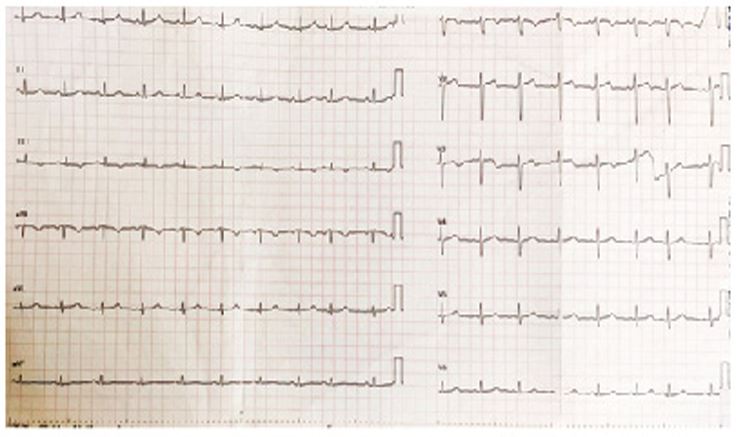
Figure 2: ECG showing sinus tachycardia with q and T wave inversion in III.
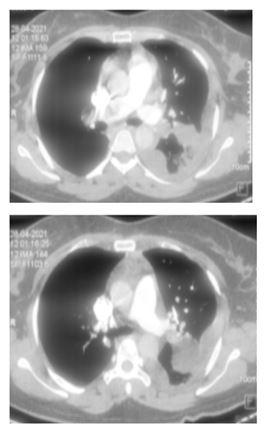
Figure 3: CT pulmonary angiogram showing thrombus in right pulmonary artery at hilum
Discussion
Multifocal venous thrombosis affecting cerebral venous sinuses and the pulmonary artery is extremely rare. It always instigates the physician to search for an underlying predisposition to recurrent thrombosis especially autoimmune disorders or thrombophilia. This middle aged lady with no obvious predisposing thrombophilia presented with venous thrombosis involving multiple sites a week following her vaccination with Covishield vaccine. In the current pandemic scenario, it is appropriate to consider both theSARS COVID19 virus as well as the recombinant vaccine developed against as potential triggers of thrombosis.
COVISHIELD or ChAdOx1 nCoV- 19 Corona Virus Vaccine (Recombinant) contains 5 × 10 replication-deficient chimpanzee adenoviral particles encoding the SARS-CoV-2 Spike (S) glycoprotein viral particles, produced in genetically modified human embryonic kidney (HEK) 293 cells [1]. It has been shown to reduce overall COVID19 infection by 70%, and severe illness by 99% [2]. However, there have been reports of associated increased risk of systemic venous thrombosis following use of these vaccines. The pathophysiology has been stated to be the formation of anti-platelet antibodies, as a part of the immune reaction, which mimics Heparin Induced Thrombocytopenia (HIT) [3,4]. The commonest site of thrombosis reported is the cerebral venous sinuses, usually 6-14 days after vaccination. Considering the overwhelming protective efficacy, the risk of thrombosis in very miniscule (0.0004 – 0.001%) [5]. It is mandatory to rule out secondary causes before shifting the blame to the vaccine. Routine thromboprophylaxis after vaccination is not recommended but strict vigilance is to be maintained for diagnosing thrombotic disorders. Treatment of any such episode is conventional anticoagulation; heparin is avoided especially if anti PF4 antibodies are positive.
Wolf et al reported 3 women with mean age 34.6 years, who presented with cerebral sinovenous thrombosis after an average of 12.3 days of administration of ChAdOx1 nCoV- 19 Corona Virus Vaccine. All of them had thrombocytopenia with positive anti PF4 and COVID19 antibodies with negative thrombophilia profile, and were treated successfully with anticoagulation alone [6]. See et al also reported 12 patients who developed CSVT 6-15 days after receiving ChAdOx1 nCoV- 19 Corona Virus Vaccine, along with documented thrombocytopenia and positive anti PF4 antibodies. Mortality rate was reported to be as high as 25% [7].
However, our patient did not have any evidence of thrombocytopenia, and COVID19 antibodies were negative. Thrombophilia workup was negative, and anti-PF4 antibodies could not be done in view of limited resources. The temporal association with CoviShield vaccine was significant, with no other risk factor for recurrent life-threatening thrombosis. It is intriguing to note that the pulmonary embolism occurred while the patient was on regular anticoagulation with rivaroxaban. Although patient underwent systemic thrombolysis and was on therapeutic anticoagulation with heparin, we could not document resolution of the thrombus in pulmonary arteries. A repeat attempt at thrombolysis or mechanical removal of thrombus were the options available. However, the patient was too sick with RV dysfunction and succumbed to the illness.
In conclusion, although the thrombotic risk of ChAdOx1 nCoV- 19 Corona Virus Vaccine is significantly less as compared to the protective benefits, it can precipitate life-threatening thromboses in a handful of patients which need to be diagnosed early with a high degree of suspicion.The appropriate anticoagulation regimen in this cohort of patients needs to be established.
Declaration
Conflicts of interest: None.
Funding: None.
References
- Ramasamy MN, Minassian AM, Ewer KJ. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021; 396: 1979-1993.
- Voysey M, Costa Clemens SA, Madhi SA. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021; 397: 881-891.
- Østergaard SD, Schmidt M, Horváth-Puhó E, Thomsen RW, Sørensen HT. Thromboembolism and the Oxford-AstraZeneca COVID-19 vaccine: side-effect or coincidence? Lancet. 2021; 397(10283): 1441-1443
- Oldenburg J, Klamroth R, Langer F, Albisetti M, von Auer C, Ay C, et al. Diagnosis and Management of Vaccine-Related Thrombosis following AstraZeneca COVID-19 Vaccination: Guidance Statement from the GTH. Hamostaseologie. 2021.
- European Medicines Agency COVID-19 vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets. 2021.
- Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and Intracranial Venous Sinus Thrombosis after "COVID-19 Vaccine AstraZeneca" Exposure. J Clin Med. 2021; 10(8): 1599.
- See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al. US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, 2021. JAMA. 2021: e217517.

