Research Article - Volume 3 - Issue 1
Elevated mean platelet volume may predict vaso-occlusive complications in type (II) diabetes mellitus
Bashir Abdrhman Bashir*
Department of Hematology, Faculty of Medical Laboratory Sciences, Port Sudan Ahlia College, Sudan.
Received Date : Nov 17, 2022
Accepted Date : Jan 11, 2023
Published Date: Feb 01, 2023
Copyright:© Bashir Abdrhman Bashir 2023
*Corresponding Author : Bashir Abdrhman Bashir, Associate Professor of Hematology, Chairman of Hematology Department Faculty of Medical Laboratory Sciences, Port Sudan Ahlia College, Port Sudan, Sudan.
Email: bashirbashir17@hotmail.com
DOI: Doi.org/10.55920/2771-019X/1363
Abstract
Introduction: Diabetes is an international metabolic pandemic condition. In this metabolic issue, increased platelet activity may play the role of mean platelet volume (MPV) as a risk factor for the development of vascular disease.
Aim: The issue of the study was tofind out the MPV in type II diabetic Sudanese patients contrasted with nondiabetics.
Materials/Methods: From August 2020 to December 2021, cross-sectional descriptive research was conducted. Platelet indices were calculated in 151 diabetic patients with type II diabetes and 57 non-diabetic participants. HbA1c and postprandial blood glucose levels were likewise estimated.
Results/ discussion: The MPV in diabetic patients was significantly augmented (9.75±0.84 fl) compared to the non-diabetics (9.0±0.49 fl; P< 0.001). MPV levels > 9.0 fl in diabetic patients were statistically significant with diabetic patients with Vaso-occlusive complications (P=0.023). The area under the ROC curve was 0.706(95% CI: 0.628 – 0.785, P = 0.000)exhibiting that MPV is an independent predictor of diabetic Vaso-occlusive events. The Kaplan-Meier model noted that the overall survival of diabetic patients with Vaso-occlusive complications with MPV > 9.0 fl was significantly lower than those with MPV ≤ 9.0 fl (log-rank p < 0.034).
Conclusions: Platelets in diabetic patients are hyperactivated, aggregable, and associated with increased MPV. Furthermore, in type II diabetes individuals, increased MPV may denote a high risk of Vaso-occlusive problems.
Keywords: Diabetes mellitus; platelet activity; mean platelet volume; sudan.
Introduction
Diabetes mellitus (DM) is a significant worldwide well-being problem [1]. According to evaluations of the World Health Organization, there were 422 million individuals experiencing diabetes worldwide in 2014 [2]. The widespread platelet activity is underlined to contribute to forming vascular disorders of this metabolic condition [3]. Platelet volume, a hallmark of the thrombocyte function and activation, is estimated as the average platelet volume (MPV) by hematological analyzers. Patients with diabetes have agreater risk for micro-and macro-vascular diseases, and platelets could be included as a causal agent for changes in platelet morphology and function [4]. The distinction in platelet volume is vividly connected with contrasts in density, dense body content, enzymatic action of lactate dehydrogenase, platelet accumulation to adenosine diphosphate (ADP), and serotonin take-up and release, which may support the significance of the MPV as anindicator of platelet function [3]. For the most part, typical platelet count fluctuates between the range of 150 x 109/l and 400 x 109/l, and ordinary platelet size (mean platelet volume) shifts somewhere in the range of 9.0 and 13.0 fl [5]. A few authors express that there is no significant distinction among an age cohort for MPV in solid subjects, though, some different surveys show a slight increment in the MPV as an age-related increase in both men and women [6]. Scarcely other different investigations uphold the possibility that youthful platelets, apparently those that were lately delivered from the bone marrow were bigger, denser, and showed few adjustments in function when contrasted with smaller platelets [7].
Aim
The goal of this study was to determine the MPV in diabetics and non-diabetics alike. The MPV of diabetics with and without vascular entrapments is being studied to see if there is a difference in qualification, as well as to anticipate the relationship between MPV and postprandial blood glucose, glycosylated hemoglobin (HbA1c), and disease duration in diabetic patients.
Material/Methods
151 known type II diabetic patients and 57 non-diabetic, healthy appearing participants were used as controls in a cross-sectional descriptive study conducted in Dr. Awaad's medical center (private sector) between August 2020 and December 2021. All the diabetic and nondiabetic subjects underwent a comprehensive clinical assessment that included a detailed examination of any underlying macro- or microvascular complications. These vascular annoyances were both microvascular (retinopathy, nephropathy, and neuropathy) and macrovascular (Stroke, diabetic septic foot, coronary heart disease, cerebrovascular, and heart failure) Of the 151 diabetic patients, 89 diabetic individuals developed Vaso-occlusive consequences, while the other 62 did not. Using a three-part differentiated semi-automated Hematology analyzer (Sysmex KX 21N, Japan), all subjects had their mean platelet volume (MPV), platelet count (PLT), platelet distribution width (PDW), plateletcrit (PCT), and platelet large cell ratio (P-LCR) estimated.
Inclusion and exclusion
This study enlisted the participation of all individuals with type II diabetes. Subjects who are unwilling to participate, pregnant women, people who are taking anticoagulant or antiplatelet drugs, people who have had a previous or current cancer, liver disease, combination of diseases, blood or blood product transfusions, and people who have nutritional anemia are all excluded from this study (nutritional anemia affect the MPV by the presence of reactive thrombocytosis or impact falsely on the HbA1c). An arrangement of individuals with a high or low MPV chose a cutoff estimate of 9.0 fl.
Definition of Vaso-occlusive complication
The complication is thought to be present when a blood vessel becomes narrow and diminishes. This can be owing to arteriosclerosis or blood vessel spasms. In arteriosclerosis, occlusion occurs within vessels and restricts the flow of blood and oxygen from organs and limbs [4].
Laboratory sample and methods
From the antecubital vein 5 ml blood specimens were gathered in di-potassium ethylene diamine tetraacetic acid (K2-EDTA), run immediately to minimize the variations due to anticoagulanteffect, and subjectto the measurement of Hb A1c. Tests were held up at room temperature. Other parts of the samples were treated with sodium fluoride to estimate the blood glucose by a glucose oxidase technique using a semi-automated chemistry analyzer (URIT-810, China). HbA1c was performed using fluorescence immune-chromatography (Wondfo, Finecare, China).
Quality control
Standard operating procedures (SOPs) and manufacturer’s directives were internationally followed, and the methods and all reagents were stored and arranged as per the manufacturer's directions. The laboratory quality nature of each testing strategy was checked by running control materials previously investigated for the platelet indices, blood glucose, and HbA1c samples. The assessment of patients' tests was done when the systems passed the quality control checking.
Statistical evaluation
Data were applied to a statistical package for the social sciences software (SPSS version 24, Chicago, IBN, USA) for analytic thinking. Nonparametric tests, Mann-Whitney test, and Wilcoxon testwere delegated for theexamination of platelet parameter boundaries between gatherings. The outcomes were presented as a mean ± standard deviation (SD). A Chi-square test has been performed for categorical data. Overall survival was conferred by the Kaplan–Meier approach to explore the cumulative incidence attribution of high and low MPV in diabetes and the statistical significance was compared with the log-rank test. Receiver operating characteristic (ROC) curves were sorted outfor quantitative variables to specify the best cutoff point values depending on the MPV area under the curve (AUC) at 95% confidence intervals. Tables were used to display the summed-up data. In all data investigations, a P. value under 0.05 was perceived as statistically significant
Results
A total of 208 members were selected and fulfilled the measures of the study. Among151 diabetic patients, 100 (66.2%) were males, and 51 (33.8%) were females with a mean age of 50.9±14.9 years. Among 57 non-diabetic individuals, 44 (77.2%) were males, and 13(22.8%) were females with an average age of 32.7±14.0 years. The analyzed parameters' potential distinctive baselines were shown in (Table 1). The mean span of diabetes was 9.5±8.2 years.
In this study, diabetic patients in Sudan had a substantially higher MPV (9.75±0.84 fl) versus the non-diabetic category (9.0±0.49 fl; P< 0.000). Moreover, the mean PLT and PCT levels were also significantly higher in diabetic patients (263.6±87.8 x109/l, 0.25±0.07 %, respectively) than non-diabetic individuals (218.2±67.6 x109/l, 0.20±0.06 %, respectively). Diabetic patients have considerably lower mean PDW and P-LCR levels than non-diabetic participants (Table 1). An arrangement of individuals with a high or low MPV chose a cutoff estimate of 9.0 fl. Out of 151 diabetic patients, 132 (87.4%) had MPV > 9.0 fl and 19 (12.6%) had MPV 9.0 fl, as determined by MPV.The median survival time (95% CI) was 17 (12.9 – 21.0) months for diabetic patients with MPV > 9.0 fl (n= 132) and 39.1 (29.7 – 48.5)months for diabetic patients with MPV < 9.0 fl (n= 19), respectively(log-rank, P < 0.034). There was a notable separation in the Kaplan-Meier curves of diabetes patients with high and low MPV (Figure 1). A positive statistical result was found among Sudanese diabetes patients. Between an MPV and diabetes duration (P = 0.004), HbA1c levels (P = 0.040), sex (P = 0.009), PDW levels (P = 0.000), P-LCR levels (P = 0.000), PCT levels (P = 0.010), and PPBG levels (P = 0.040), Spearman's correlation was highlighted. However, there was no statistical link found between MPV and PLT levels, age, and Vaso-occlusive problems. Table 2 displays the link between clinical baseline parameters of Sudanese diabetic patients and MPV levels. The MPV 9.0 fl. was bound to show high PLT levels. MPV > 9.0 fl were found to have higher MPV, PDW, PCT, and P-LCR levels (Table 2).
89 (58.9%) of 151 diabetic individuals experienced Vaso-occlusive problems, while 62 (41.1%) did not. Diabetic patients with and without Vaso-occlusive problems had their platelet indices compared. In diabetic patients with Vaso-occlusive consequences, MPV levels > 9.0 fl were statistically significant (P = 0.016) (Table 3).
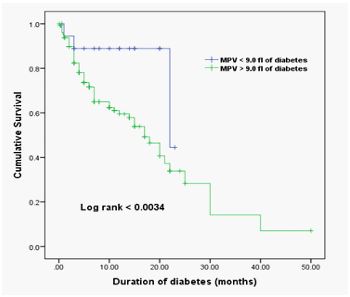
Figure 1: Kaplan–Meier Analysis of the Cumulative Incidence of diabetes at high and low MPV averages around the cut-off of 9.0 fl. The median survival time (95% CI) was 17 (12.9 – 21.0) months for 132 diabetic patients with MPV > 9.0 fl and 39.1 (29.7 – 48.5) months for 19 diabetic patients with MPV < 9.0 fl, respectively
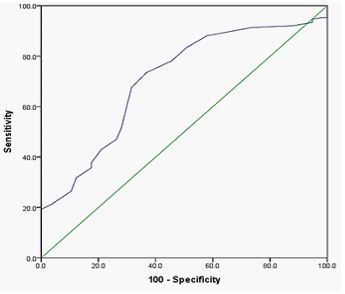
Figure 2: The optimized cut-off value was scanned for the MPV through a standard ROC curve analysis.
ROC curves were estimated using the MPV levels examined in this study for diabetic patients, and it was discovered that high MPV significantly predicted Vaso-occlusive complications (AUC= 0.706, 95% CI: 0.628 – 0.785, P = 0.000) (Figure 2). We grouped diabetic patients into two categories based on their HbA1c levels: category A (HbA1c 6.5%) and category B (HbA1c > 6.5%). Out of 151 diabetic patients, there were 31 (20.5%) diabetic patients in category A (mean 5.39±0.92 %) and 120 (79.5%) diabetic patients in category B (mean 11.18±3.7 %). The mean number of PLT levels in category A (262.2±89 x 109/l) was lower than in category B (268.9±86 x 109/l). The meanMPV levelsin category A (9.72±0.82 fl) wereinsignificantly reduced compared to that of category B (9.87±0.93 fl; P = 0.702).
Table 1: Outcomes of study parameters between diabetic and non-diabetic individuals.
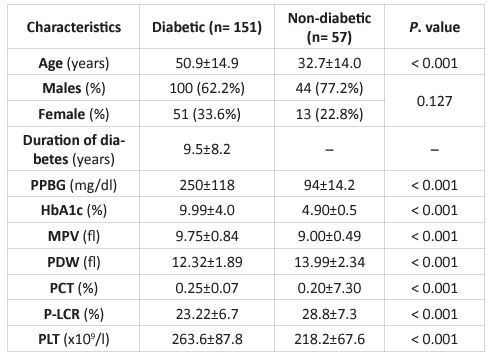
PPBG; postprandial blood glucose, MPV; mean platelet volume, HbA1c; hemoglobin A1c, PDW; platelet distribution width, PCT; plateletcrit, P-LCR; platelet large cell ratio, PLT; platelet count.
Table 2: Relation between the clinical parameters and MPV levels
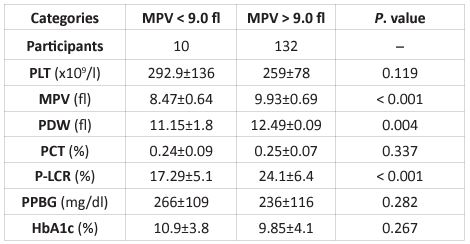
PPBG; postprandial blood glucose, MPV; mean platelet volume, HbA1c; hemoglobin A1c, PDW; platelet distribution width, PCT; plateletcrit, P-LCR; platelet large cell ratio, PLT; platelet count.
Table 3: Comparison of findings among diabetics with and without Vaso-occlusive complications
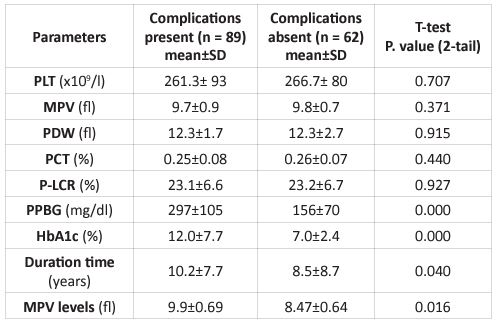
PPBG; postprandial blood glucose, MPV; mean platelet volume, HbA1c; hemoglobin A1c, PDW; platelet distribution width, PCT; plateletcrit, P-LCR; platelet large cell ratio, PLT; platelet count.
Discussion
Diabetes mellitus is a creeping metabolic anomalymarked by constant hyperglycemia,which leadsto issues affecting the peripheral nerves, eyes, kidneys, and micro and macrovascular clog [3]. Nations with an overwhelming number of diabetic patients are China (116.4 million), India (77 million), the United States (31 million), Pakistan (19.4 million), and Brazil (16.8 million) [8]. The incidence of diabetes-related microvascular difficulties is increased in individuals with poor glycemic control, a lengthy span of diabetes, associated hypertension, as well as obesity [3]. The incidence of diabetes in Sudan is about 3.9%, which thus leads to an expansion in the proportion of admission [9]. A lower limb is lost every 30 seconds as a result of vascular problems [10]. Thrombotic events account for 80% of diabetes fatality rates. Whereas 75% of these deaths were caused by cardiovascular problems, with the remaining 25% attributed to peripheral vascular troubles and cerebrovascular events [9].
Diabetic Mellitus and associated Vaso-occlusive consequences can encounter financial devastation and impact negatively on national economies. As a result, the MPV value can be used as a simple and cost-effective parameter in the evaluation and monitoring of diabetes, helping to repression of morbimortality [3]. MPV is a fraction of the magnitude and activity of thrombocytes on average. Giant platelets are more reactive, hyperactive, and aggregable than regular platelets [11]. As a result, MPV can expose the pro-coagulant impact and event of thrombotic vascular complications. This establishes a link between the MPV and diabetic Vaso-occlusive outcomes, implying that MPV fluctuation could reflect thrombogenesis [3]. Due to the rupture of atherothrombotic plaques, there may be little bleeds, which can lead to increased platelet recruitment, powerful reactivity, and marrow promotion. The high MPV index has been emanated as a new surrogate risk factor for diabetic vascular troubles [11]. Diabetes has been labeled as a prothrombotic condition due to platelet hyperactivity [9]. Platelet reactivity can be boosted by hyperglycemia, which causes non-enzymatic glycation of proteins on the platelet's surface, as well as the osmotic effect of glucose and the activation of protein kinase C. The fluidity of the membrane is reduced, and the platelet's propensity to activate is increased [12]. Platelets with diabetes have dysregulated signaling shunts, which contribute to increased activation and aggregation in response to a specific stimulus (hyper-reactivity platelet) [11].
The findings from this study, which resemble the findings of Jindal et al [13] and Kodiatte et al [3], suggest that PLT and MPV were higher in diabetics than in non-diabetics.Additionally, patients with diabetes who had Vaso-occlusive complications had MPV levels > 9.0 fl. These findings were also observed in studies conducted by Change et al and Kodiatte et al [3,11]. This suggests the significant contribution of hyper-reactive platelets in the pathogenesis of vascular disorders. In contrast, MPV was statistically insignificant in patients with or without vascular disorders in a study by Demirtune et al [14]. Dissimilarities may be attributed to different numbers of patients or other vascular disorders. We discovered, in our study, that MPV levels are significantly related to clinical parameters in diabetic patients and predict Vaso-occlusive complications. The association between HbA1c and MPV levels was also insignificant, which contradicts the findings of another study by Demirtune et al.Thusly, it may be presumed that glycemic control diminishes the hyper-reactivity of the plateletsand consequently may forestall or delay conceivable diabetic Vaso-occlusion. Moreover, there was an MPV association seen with theduration of diabetes. A finding is consistently similar to Ateset al [15]. However, our findings were in contrast to those of Kodiatteet al this may be due to our limited sample size. In their study, Demirtaset al [16] and Shilpi et al [17] found that diabetics with vascular difficulties had vastly higher MPV than diabetics without vascular complications. This revealed that enhanced platelet activity has a significant impact on the pathogenesis of vascular problems.
Limitations
Our research will serve as a foundation for future research and will eventually aid in the diagnosis and treatment of diabetes individuals. Furthermore, our findings must be confirmed in larger investigations. Qualitative platelet function tests and other explanations of increased platelet volume were among the examination's limitations that could not be explored. Furthermore, no cardiovascular check was assessed.
Conclusion
Platelets in Sudanese diabetic individuals become hyperactive, aggregable, and have a higher MPV, according to this study. Furthermore, a substantial risk of Vaso-occlusive problems may be associated with higher MPV. As a result, MPV could be a valuable predictor of diabetic Vaso-occlusive problems. This study also looked into whether a high MPV was linked to a higher HbA1c index. Increased MPV levels, on the other hand, are thought to be a sign of Vaso-occlusive problems, which may need to be confirmed.
References
- Mahsud MAJ, Khan A, Hussain J. Hematological Changes in Tobacco using Type 2 Diabetic Patients. Gomal J Med Sci. 2010; 8: 8-11.
- World Health Organization. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/ [Last accessed on 2020Aug27].
- Kodiatte TA, Manikyam UK, Rao SB, Jagadish TM, Reddy M, et al. Mean platelet volume in type 2 diabetes mellitus. J Lab Physicians. 2012; 4: 5-9.
- Radha RK, Selvam D. MPV in Uncontrolled & Controlled Diabetics- Its Role as an Indicator of Vascular Complication. J Clin Diagn Res. 2016; 10(8): EC22-EC26.
- Operator’s manual automated hematology analyzer. Introduction, analysis parameters. Sysmex. Japan. 2006; 1-5.
- Demirin H, Ozhan H, Ucgun T, Celer A, Bulur S, Cil H, et al. Normal range of mean platelet volume in healthy subjects: Insight from a large epidemiologic study. Thromb Res. 2011; 128(4): 358-60.
- Lippi G, Meschi T, Borghi L. Mean platelet volume increases with aging in a large population study. Thromb Res. 2012; 129(4): e159-60.
- Statistica. Countries with the highest number of diabetics worldwide in 2019. https://www.statista.com/statistics/281082/countries-with-highest-number-of diabetics/#:~:text=Number%20of%20people%20with%20diabetes%2C%20by%20country%202019&text=China%20is%20the%20country%20with,134%20million%20people%20with%20diabetes. [Last accessed 29 august 2020].
- Bashir AB, Ali MS. Hemostatic state augmented with platelet indices among Sudanese diabetic septic foot. BMC Hematology. 2018; 18: 11.
- Rassouli B, Ghyour MB, Ghyour N. Microvascular complication of diabetes. J Biol Sci. 2010; 10: 411-23.
- Chang HA, Hwang HS, Park HK, Chun MY, Sung JY. The Role of Mean Platelet Volume as a Predicting Factor of Asymptomatic Coronary Artery Disease. Korean J Fam Med. 2010; 31: 600-6.
- Kakouros N, Rade JJ, Kourliouros A, Resar JR. Platelet function in patients with diabetes mellitus: from a theoretical to a practical perspective. Int J Endocrinol. 2011; 742719.
- Jindal S, Gupta S, Gupta R, Kakkar A, Singh HV, Gupta K, et al. Platelet indices in diabetes mellitus: indicators of diabetic microvascular complications. Hematology. 2011; 16: 86-9.
- Demirtunc R, Duman D, Basar M, Bilgi M, Teomete M, Garip T. The relationship between glycemic control and platelet activity in type 2 diabetes mellitus. J Diabetes Complications. 2009; 23: 89-94.
- Ateş O, Kiki I, Bilen H, Keleş M, Koçer I, Kulaçoğlu DN et al. Association of Mean Platelet Volume with The Degree of Retinopathy in Patients with Diabetes Mellitus. Eur J Gen Med. 2009; 6: 99-102.
- Demirtas L, Degirmenci H, Akbas E, Ozcicek A, Timurgglu A, et al. Associattion of hematological indiceswith diabetes, impaired glucose regulation and microvascular complications of diabetes. Int J Clin Exp Med. 2015; 8: 11420-11427.
- Shilpi K, Potekar RM. A study of platelet indices in type 2 diabetes mellitus patients. Indian J Hematol Blood Transfus. 2018; 34 (1): 115-120.

