Research Article - Volume 3 - Issue 1
Host epigenetics- Gut microbiome interactions in irritable bowel syndrome subjects during Vitamin D supplementation
Mostafa Fazeli1; Mohammad Mohammad-Zadeh2; Seyed-Amir Tabatabaeizadeh3; Gordon Ferns4; Majid Gayour Mobarhan5; Zahra Meshkat6*
1Department of Medical Genetics and Molecular Medicine, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
2Cellular and Molecular Research Center, Sabzevar University of Medical Sciences, Sabzevar, Iran.
3Clinical Nutrition Department, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
4Division of Medical Education, Brighton & Sussex Medical School, Falmer, Brighton, Sussex BN1 9PH, UK.
5Biochemistry of Nutrition Research Center, School of Medicine, Mashhad University of Medical Sciences, 99199-91766, Mashhad, Iran.
6Antimicrobial Resistance Research Center, Mashhad University of Medical Sciences, Mashhad, IR Iran.
Received Date : Nov 19, 2022
Accepted Date : Jan 12, 2023
Published Date: Feb 03, 2023
Copyright:© Zahra Meshkat 2023
*Corresponding Author : Zahra Meshkat, Antimicrobial Resistance Research Center, Mashhad University of Medical Sciences, Mashhad, IR Iran.
Email: Meshkatz.mums.ac.ir@gmail.com
DOI: Doi.org/10.55920/2771-019X/1364
Abstract
There is evidence of a link between vitamin D deficiency and Irritable Bowel Syndrome (IBS), and vitamin D supplementation can help improve it, but the mechanisms of this link still are unclear. In this study, we attempted to investigate the hostepigenetic -gut microbiome interactions during vitamin D supplementation in IBS patients. For this purpose, out of 988 participants in the Vitamin D supplementation program, 154 students with IBS symptoms were identified using Rome III criteria. Finally, only 17 patients were studied for gut microbiome considering exclusion criteria. Methylation specific PCR was used for epigenetic evaluation and TaqMan Real Time PCR was used for gut microbiome assessment. The results show that the prevalence vitamin D deficiency in patients with IBS is very high and is significantly reduced by the intervention. During supplementation, the gut microbiome undergoes changes that decrease in Firmicutes and increase significantly in Bifidobacterium and Enterococcus genera. The methylation pattern of the studied genes has also changed during supplementation. Interactions have been observed between the studied bacteria and also the Lactobacillus, Bifidobacterium and Enterococcusgenera have affected the methylation changes of vdr and cd14. Inaddition, Bifidobacteriumspp and Firmicutes were effective in altering the methylation pattern of s100A9 and vdr, respectively. Based on the findings of this study, it can be concluded that epigenetic and microbial interactions in the colon during vitamin D supplementation may be involved in improvement the condition of IBS patients.
Keywords: Vitamin D deficiency; gut microbiota; IBS.
Introduction
Irritable bowel syndrome is a common functional gastrointestinal disorder that is diagnosed by Rome criteria based on some clinical symptoms including abdominal pain, bloating, and changes in bowel habits [1]. Brain-gut axis and immune system dysfunctions, abdominal hypersensitivity, and gastrointestinal disturbances are associated with IBS [2]. Its prevalence is more common among women and young adults and is between 5 and 20% [1,2]. Genetics, gastrointestinal disorders, anxiety, the intestinal-brain axisdisorders, infection and inflammation in the intestine, and changes in the gut microflora are among the causes of IBS. In IBS, genetic and environmental factors affect host-microbial interactions [3]. Recent evidence suggests that gut microbiota are an important factor in the etiology of IBS [4]. Changes in the intestinal environment have caused compositional or functional changes in the gut microbiota called dysbiosis, which is associated with IBS [5,6]. Lack of VDR can also cause dysbiosis and functional changes in the gut microbiome [7]. So far, studies have shown changes in the relative abundance of certain groups of bacteria during IBS [8]. The symptoms and severity of IBS have subsided after the use of probiotic compounds and the improvement of gut microbiota composition [9]. The gut microbiota is composed of a wide variety of microbial species that play an essential role in the evolution and development of host immune responses and also affect the physiology of the host by affecting intestinal barrier homeostasis and gastrointestinal motility [4]. Vitamin D receptors are located in the gut and have high levels of expression in the small intestine and colon [10]. The epigenetic effects of microbiome on VDR modulation have been discussed in several studies [11]. One of the functions of the active form of vitamin D in the body is to regulate inflammation [1,10,12]. The prevalence of vitamin D deficiency is higher in IBS patients than in the healthy population, and the severity of symptoms is inversely related to serum vitamin D levels [12,13]. Vitamin D supplementation has been shown to improve IBS symptoms and improve the quality of life of patients [2,14]. Also, many studies have reported the effect of vitamin D supplementation on changes in gut microbiota [10,15,16]. Genomic wide association studies has identified variations in VDR that affect changes in gut microbiota [17]. Decreased vitamin D levels and decreased VDR regulation are associated with intestinal dysbiosis [18]. Vitamin D deficiency leads to changes in the gut microbiome, which in turn reduces the production of vitamin B in the intestine [19]. It should be noted that the mechanism of action of vitamin D on IBS symptoms has not been elucidated in recent trials [1-3,14]. We aimed to investigate host epigenetic- gut microbiome interaction during vitamin D supplementation to clarify how vitamin D improve IBS symptoms.
Material/Methods
This study is an experimental study of intervention before and after the intervention (code 940849) as a vitamin D supplement with a dose of 50,000 IU (vitamin D supplement supplementation plan with code 931188) for nine weeks in a population of 988 female students (11 to 16 years old). According to Rome III criteria 154 of them met the criteria and diagnosed as IBS. Finally, taking into account the exclusion criteria, including irregular use of vitamin supplements, use of any type of antibiotic at least two weeks after sampling, and undesirable macroscopic characteristics of the stool sample; Only 17 participants were included in the study to evaluate the gut microbiome and the epigenetic status of colon genes. This study has been approved by the ethics committee of Mashhad University of Medical Sciences. The Rome III questionnaire was used to diagnose IBS in participants and its validity was previously reported. According to Rome III criteria, IBS considered as condition with recurrent abdominal pain at least three days a month for at least the past six months with two or more symptoms such as improved bowel movements, onset with changes in shape or number of bowel movements. Stool samples were taken from participants before and after the intervention. Stool samples immediately transferred to laboratory in icebox. DNA extraction was performed from fecal samples by using QIAamp DNA stool mini kit (Qiagen, Germany).
In this study, some conserved genes of three genera and two bacterial phyla were detected and analyzed by TaqMan Real Time PCR in the genome extracted from fecal samples. The primers and probes used to detect these bacteria are listed in (Table 1). The tuf gene in Lactobacillus spp. and the 16S rRNA gene in theFirmicutes and, Bacteroidetes phyla [20] and, Bifidobacteriumspp [21] and the 23S rRNA gene in the genus Enterococcus [22] were detected. The standard curve for estimating the amount of detected bacteria in fecal samples was plotted by RealTime PCR. To draw a standard curve, DNA dilution extracted from standard bacteria was used and compared with the culture results of these bacteria. Clostridium perfringens PTCC 1765, Bacteroidesfragilis ATCC 23745, Bifidobacterium bifidum PTCC 1644, Entrococcusfaecalis IBRC-M 11130 and Lactobacillus casei IBRC-M 10711 were cultured on FAA medium at 37 °C for 24 hours. Colony counts were performed to calculate CFU and compared with the results of quantitative real-time PCR analysis of DNA extracted from standard bacteria.
Due to the epigenetic effects of microbes on host genes and also the possibility of regulating genes involved in vitamin D function by DNA methylation, in addition to vdr [23], the methylation pattern of some genes related to vitamin D immune function including nlrp3 [24], cd14, nod1 and S100A9was examined using Methylation method specific PCR. To design MSP primers, EPD (Eukaryotic Promoter Database) was used to extract the upstream sequence of the studied genes [25]. Then, Meth Maker software was used to design specific MSP primers [26]. All data were analyzed using SPSS software package version 20. In order to perform the correlation test between before and after the intervention, the normality of the observations were tested by two Kolmogrov-Smirnov with Liliforces corrections and Shapiro-Wilk test.
Demographic information was expressed as Median (IQR3- IQR1) and Mean ± SD for data with abnormal and normal distribution, respectively, and the frequency of overweight and obesity and methylation states were reported as a percentage. Statistical analyzes were performed between two groups of variable type using X2 and t-pair tests. Significance level in all statistical tests was considered at p-value level less than 0.05 error level. The correlation of variables by Pearson, Spearman r and Point-biserial methods was examined for qualitative, quantitative and qualitative-quantitative variables, respectively. For observations that are not normally distributed before and after the intervention (p-value less than the error level of 0.05 indicates no normal distribution). Therefore, Spearman r correlation coefficient was used to calculate the correlation coefficient. In statistical modeling, the normality of the residual distribution was investigated using Kolmogrov-Smirnov with Liliforces corrections and Shapiro-Wilk tests as well as the qq-plots. To convert the abnormal distribution to normal, the box-cox conversion has been used, and in cases where this conversion has not been effective, weighted cases have been used for statistical modeling. In finally modeling conducted by weighted least squared regressions.
Results
The prevalence of deficiency in participants with IBS is shown in (Table 2). Serum levels of vitamin D before and after the intervention were measured 6.4 ng/mL (10.5 -3.6) and 38.2 ng/mL (49.7 -26.4), respectively, which indicates significant changes in serum vitamin D levels in people with IBS after the intervention. Examination of the frequency of vitamin D deficiency in people with IBS shows that the prevalence of severe deficiency in them is very high, which has sharply decreased after intervention.
The frequency of epigenetic states of the studied genes before and after the intervention is shown in (Table 3). The promoter methylation of cd14, nlrp3 and s100A9 genes was increased, while no change was observed in vdr and nod1methylated promoters. Microbial abundance before and after the intervention was measured by taqMan Real Time PCR and is shown in (Table 4). The population of the bacterial phylum,Firmicutes in individuals with IBS after the intervention showed a significant decrease. Investigation of the interaction relationships between gut microbiome and epigenetics shows that the population changes of Lactobacillus spp. andBacteroidetes have a significant effect on population changes of Enterococcusspp., also the interaction of these two microbial groups is significant on Enterococcusspp. changes (Figure 1). The effect of demographic changes in the studied bacteria on the epigenetic status of the studied genes has shown that only the Bacteroidetesphylum had no significant effect on the changes in the studied genes (Figure 2).
Table 1: primers and probes for detection of bacterial species and phylum.
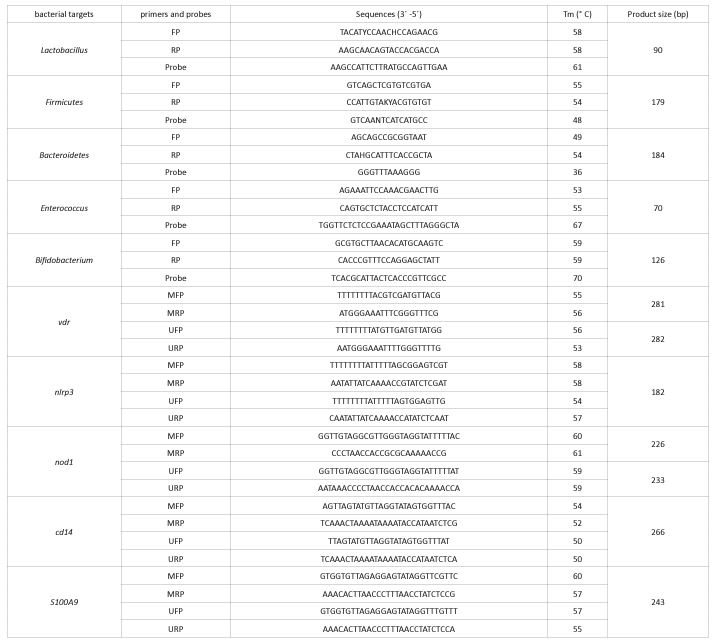
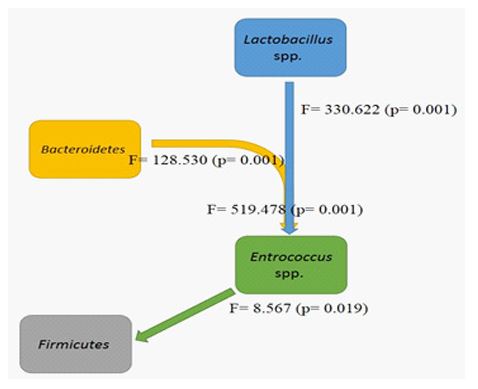
Figure 1: Effects of interactions of gut microbiome components on each other during vitamin D supplementation
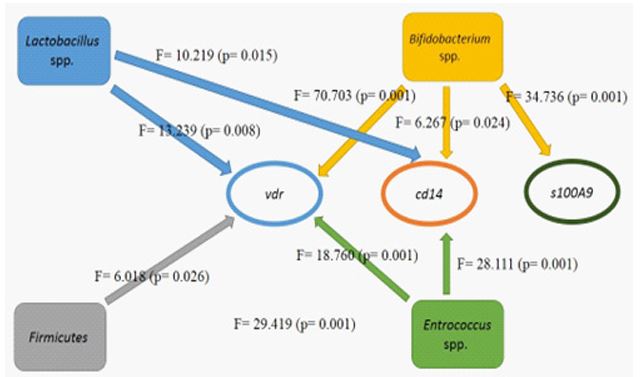
Figure 2: Effects of microbiome changes on alteration in the methylation pattern of vitamin D-related genes during vitamin D supplementation.
Table 2: Frequency of vitamin D deficiency in people with IBS
Table 3: Frequency percentage of various methylation n state

Discussion
To our knowledge, this is the first report to examine the interaction of the gut microbiome and the epigenetic status of vitamin D-related genes in patients with IBS. Vitamin D deficiency and severity are higher in patients with IBS than in their healthy counterparts [12], and in most cases these patients respond favorably to vitamin D supplementation [1,3,14,27]. There is also evidence that the composition of gut microbiota in subjects with IBS is different from that of normal individuals [8,28,29]. The available data also suggest that vitamin D affects the evolution of the gut microbiome [10,15], and that the gut microbiome is able to regulate the function of host genes through epigenetic mechanisms.
This study investigates changes in the methylation pattern of some genes associated with vitamin D involved in the regulation of gut microbiota ecology. The results show that the serum level of vitamin D in people with IBS after supplementation has significantly increased and the prevalence of vitamin D deficiency has also decreased due to vitamin D supplementation. Complete improvement in symptoms was observed in 23 of 154 participants after vitamin D supplementation. Studies of changes in the gut microbiome showed that the Firmicutesphylum and the Lactobacillusspp decreased significantly, while the Bifidobacterium spp showed the opposite trend. Examination of gut microbiome interactions during vitamin D supplementation has shown that changes in members of Bacteroidetes and Lactobacillusspp. have significant effects on changes in Enterococcusspp and consequently changes in Enterococcus spp. have also affected changes in Firmicutes. The study of changes in gene methylation pattern also showed that the percentage of methylated promoters in cd14, nlrp3 and s100A9 genes increased while no change was observed in vdr and nod1 genes, however the percentage of semi-methylated promoters in both genes. Investigation of the interactions of gut microbiome changes and epigenetic genes has shown that changes in the Enterococcus spp., Lactobacillusspp and Bifidobacterium spp are effective in changing the methylation pattern of vdr and cd14. In addition, vdr has been affected by changes in the Firmicutesphylum. Changes in the Bifidobacterium spp were also effective in altering the methylation pattern of the inflammatory marker gene of the colon, s100A9.
In this study, the prevalence of vitamin D deficiency in participants with IBS was very high, and similar studies have reported a high prevalence of vitamin D deficiency in patients with IBS [12,14,27]. These similarities suggest that vitamin D deficiency may be a risk factor for IBS. Numerous studies have also shown the effectiveness of vitamin D supplementation in improving IBS, which has also been observed in this study [1-3,14,27]. However, so far the mechanisms of complementarity have been unclear. In this study, an attempt was made to investigate the genetic interactions of the host with the evolution of the gut microbiome as possible mechanisms of effectiveness.
Previous studies have shown a higher abundance of Firmicutes in people with IBS compared to their natural counterparts [30,31]. In this study, the Firmicutes has a relatively higher abundance than the Bacteroidetes and also has a significant decrease in population during vitamin D supplementation. These changes may be one of the factors in improving clinical symptoms in patients with IBS due to vitamin D supplementation. In present study, it was found that changes in the Firmicutesphylum were directly and indirectly affected by the Enterococcusspp and Lactobacillusspp., respectively. Both genera have members that exhibit probiotic properties and are used in most probiotic formulations [9]. Perhaps one of the effects of probiotics in improving IBS symptoms is their effect on the changes of the Firmicutes. Also, changes in the Bacteroidetes Affected on Enterococcusspp can indirectly affect changes in the Firmicutes. These interactions confirm that an imbalance between the microbial populations of the Bacteroidetes and Firmicutesphyla, previously reported in several studies, may be a contributing factor to dysbiosis and IBS symptoms.
The study of methylation pattern during vitamin D supplementation in subjects with IBS has not been performed so far and the present study is the first report in this regard. Methylation changes have been observed in all genes studied. Only two genes, vdr and nod1, did not show any change in the percentage of their methylated promoters. However, the percentage of unmethylated promoters decreased and the semi-methylated increased. These two genes appear to be negatively regulated during vitamin D supplementation. As the serum level of vitamin D increases or certain changes in the gut microbiome decrease. Targeted vdrdeletion in intestinal cells alters the gut microbiome, leading to an increased risk of obesity [32]. In this study, we observed increasing in methylation level of vdr promoter after vitamin D supplementation which that may be lead to decreased expression, and methylation state showed the relationship with gut microbiome alteration. These results suggested that VDR play the critical role in immunity system regulation in faced with gut microbiota ecology. Decreased intestinal VDR leads to an increase in amino acid metabolites that play a protective role against oxidative stress and inflammation [32,33]. In present study after vitamin D supplementation, increased methylation level of vdr and improvement of IBS symptoms have been observed. These observations may be related to metabolite profile changing in gut and decreasing inflammatory processes. Targeted vdrdeletion may lead to the accumulation of bile acids, which are important factors in changes in the gut microbiome in obese mice [34]. The gut microbiome alteration during vitamin D supplementation have been occurred which accompanied with increasing methylation level of vdr gene, this methylation may be lead to decreasing expression level of vdr and subsequently bile acids accumulation.
The nod1 is a sensor for bacterial peptidoglycans and appears to have undergone negative promoter methylation changes during vitamin D supplementation, which may eventually lead to an increase in the population of gram-negative and positive bacteria, including the Bacteroietesphylum, which is less common in compared to normal individuals in patients with IBS, these changes can also prevent further population decline in the Firmicutesphylum and ultimately contribute to balance in the gut microbiota. Along with TLRs, the cd14co-receptor acts as a sensor for bacterial derivatives such as LPS and helps regulate microbial ecology by launching immunological cascades. In this study, it was found that more promoters of cd14 became unmethylated during vitamin D supplementation. Further activation of cd14 may lead to the identification and thus greater control of the population of gram-negative groups such as Bacteroietes, which will ultimately control the population of this bacterial group; Creating this balance may help improve patients' symptoms. On the other hand, changes in cd14 methylation pattern have been influenced by changes in the Enterococcus spp., Lactobacillus spp. and Bifidobacterium spp. The first two genera have directly and indirectly affected Firmicutes changes. The N-acetylmuramate released from Lactobacillus acidophilus had an anti-inflammatory effect on LPS-induced inflammation [35]. Lactobacillus spp.alteration effected on cd14 methylation pattern which may be possible mechanism ofanti-inflammatory effect of L. acidophilus on LPS-induced inflammation.
Another gene studied in this study is nlrp3, which is involved in inflammatory pathways and ultimately helps control microbial ecology in the colon by activating immunity arms [36-38]. The findings of present study show that the percentage of methylatednlrp3 promoters increased after the intervention. It can be argued that these changes ultimately lead to a decrease in the activity of the intestinal immune system and thus pave the way for increasing the dynamics of microbial populations as well as reducing inflammatory processes in the colon.
Finally, with decreasing inflammatory levels in the colon, an increase in methylation of the s100A9 gene seems reasonable as observed in this study. The s100A9 gene encodes a subunit of the inflammatory protein cloprotectin, which may be expressed as the inflammation increase. Host-microbiota interactions during vitamin D supplementation have led to a significant increase in the population of the genus Bifidobacterium, which has previously been reported to have positive effects in improving IBS symptoms [9]. Perhaps one of the mechanisms by which these bacteria exert their positive effects is the application of hypermethylation to promoters of inflammatory genes such as s100A9.
Conclusion
Vitamin D supplementation increases serum levels of vitamin D in people with IBS, and as a result of these interventions, improvement in the condition has been observed in them. This improvement appears to be due to changes in the gut microbiota and changes in the host epigenetic patterns. As a result of complementarity, changes in the gut microbiome can be detected and the pattern of gene methylation is altered as a result of interaction with the gut microbiota. It seems that vitamin D supplementation by modulating the gut microbiome and host epigenetics can help improve the symptoms of IBS in subjects. Further studies are needed to better understand host-microbiome interactions in IBS.
Conflict of interest: There is no conflict of interest.
References
- Sikaroudi MK, Mokhtare M, Janani L, Kashani AHF, Masoodi M, Agah S, et al. Vitamin D3 Supplementation in Diarrhea-Predominant Irritable Bowel Syndrome Patients: The Effects on Symptoms Improvement, Serum Corticotropin-Releasing Hormone, and Interleukin-6–A Randomized Clinical Trial. Complementary Medicine Research. 2020:1-8.
- Abbasnezhad A, Amani R, Hajiani E, Alavinejad P, Cheraghian B, Ghadiri A. Effect of vitamin D on gastrointestinal symptoms and health‐related quality of life in irritable bowelsyndrome patients: a randomized double‐blind clinical trial. Neurogastroenterology & Motility. 2016; 28(10): 1533-44.
- Williams CE, Williams EA, Corfe BM. Effect of vitamin D supplementation on irritable bowel syndrome symptom severity and quality of life. Proceedings of the Nutrition Society. 2020; 79(OCE1).
- De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, Jury J, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Science translational medicine. 2017; 9(379).
- Chassard C, Dapoigny M, Scott KP, Crouzet L, Del'Homme C, Marquet P, et al. Functional dysbiosis within the gut microbiota of patients with constipated‐irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2012; 35(7): 828-38.
- Halkjær SI, Christensen AH, Lo BZS, Browne PD, Günther S, Hansen LH, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018; 67(12): 2107-15.
- Jin D, Wu S, Zhang Y-g, Lu R, Xia Y, Dong H, et al. Lack of vitamin D receptor causes dysbiosis and changes the functions of the murine intestinal microbiome. Clinical therapeutics. 2015; 37(5): 996-1009. e7.
- Collins SM. A role for the gut microbiota in IBS. Nature reviews Gastroenterology & hepatology. 2014; 11(8): 497-505.
- Bonfrate L, Di Palo DM, Celano G, Albert A, Vitellio P, De Angelis M, et al. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. European journal of clinical investigation. 2020; 50(3): e13201.
- Tabatabaeizadeh S-A, Fazeli M, Meshkat Z, Khodashenas E, Esmaeili H, Mazloum S, et al. The effects of high doses of vitamin D on the composition of the gut microbiome of adolescent girls. Clinical Nutrition ESPEN. 2020; 35: 103-8.
- Apprato G, Fiz C, Fusano I, Bergandi L, Silvagno F. Natural Epigenetic Modulators of Vitamin D Receptor. Applied Sciences. 2020; 10(12): 4096.
- Abbasnezhad A, Amani R, Hasanvand A, Yousefi Rad E, Alipour M, Saboori S, et al. Association of serum vitamin D concentration with clinical symptoms and quality of life in patients with irritable bowel syndrome. Journal of the American College of Nutrition. 2019; 38(4): 327-33.
- Khayyatzadeh SS, Vatanparast H, Avan A, Bagherniya M, Bahrami A, Kiani MA, et al. Serum transaminase concentrations and the presence of irritable bowel syndrome are associated with serum 25-hydroxy vitamin D concentrations in adolescent girls who are overweight and obese. Annals of Nutrition and Metabolism. 2017; 71(3-4): 234-41.
- Jalili M, Vahedi H, Poustchi H, Hekmatdoost A. Effects of vitamin D supplementation in patients with irritable bowel syndrome: a randomized, double-blind, placebo-controlled clinical trial. Int J Prev Med. 2019; 10.
- Charoenngam N, Shirvani A, Kalajian TA, Song A, Holick MF. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: A randomized, double-blinded, dose-response study. AnticancerResearch. 2020; 40(1): 551-6.
- Naderpoor N, Mousa A, Fernanda Gomez Arango L, Barrett HL, Dekker Nitert M, de Courten B. Effect of vitamin d supplementation on faecal microbiota: A randomised clinical trial. Nutrients. 2019; 11(12): 2888.
- Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nature genetics. 2016; 48(11): 1396-406.
- Singh P, Kumar M, AlKhodor S. Vitamin D deficiency in the Gulf cooperation council: exploring the triad of genetic predisposition, the gut microbiome and the immune system. Frontiers in immunology. 2019; 10: 1042.
- Gominak S. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a “pro-inflammatory” state associated with atherosclerosis and autoimmunity. Medical hypotheses. 2016; 94: 103-7.
- Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PloS one. 2009; 4(9).
- Rinttilä T, Kassinen A, MalinenE, Krogius L, Palva A. Development of an extensive set of 16S rDNA‐targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real‐time PCR. J Appl Microbiol. 2004; 97(6): 1166-77.
- He J-W, Jiang S. Quantification ofenterococci and human adenoviruses in environmental samples by real-time PCR. Appl Environ Microbiol. 2005; 71(5): 2250-5.
- Marik R, Fackler M, Gabrielson E, Zeiger MA, Sukumar S, Stearns V, et al. DNA methylation-related vitamin D receptor insensitivityin breast cancer. Cancer biology & therapy. 2010; 10(1): 44-53.
- Tang SC, Yeh JI, Hung SJ, Hsiao YP, Liu FT, Yang JH. Glycolic acid silences inflammasome complex genes, NLRC4 and ASC, by inducing DNA methylation in HaCaT cells. DNA and cell biology. 2016; 35(3): 124-34.
- Dreos R, Ambrosini G, Périer RC, Bucher P. The Eukaryotic Promoter Database: expansion of EPDnew and new promoter analysis tools. Nucleic acids research. 2015; 43(D1): D92-D6.
- Schüffler P, Mikeska T, Waha A, Lengauer T, Bock C. MethMarker: user-friendly design and optimization of gene-specific DNA methylation assays. Genome biology. 2009; 10(10): R105.
- Shi SM, Wen YL, Hou HB, Liu HX. Effectiveness of vitamin D for irritable bowel syndrome: A protocol for a systematic review of randomized controlled trial. Medicine. 2019; 98(9): e14723. [DOI:10.1097/md.0000000000014723].
- El-Salhy M, Hatlebakk JG, Hausken T. Diet in irritable bowel syndrome (IBS): Interaction with gut microbiota and gut hormones. Nutrients. 2019; 11(8): 1824.
- Öhman L, Simrén M. Intestinal microbiota and its role in irritable bowel syndrome (IBS). Current gastroenterology reports. 2013; 15(5): 323.
- Jeffery IB, O'toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012; 61(7): 997-1006.
- Rajilić–Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011; 141(5): 1792-801.
- Chatterjee I, Lu R, Zhang Y, Zhang J, Dai Y, Xia Y, et al. Vitamin D receptor promotes healthy microbial metabolites and microbiome. Scientific Reports. 2020; 10(1): 1-18.
- Niu X, Zheng S, Liu H, Li S. Protective effects of taurine against inflammation, apoptosis, and oxidative stress in brain injury. Molecular medicine reports. 2018; 18(5) :4516-22.
- Zheng X, Huang F, Zhao A, Lei S, Zhang Y, Xie G, et al. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC biology. 2017; 15(1): 1-15.
- Wu Z, Pan D, Guo Y, Zeng X. N-acetylmuramic acid triggers anti-inflammatory capacity in LPS-induced RAW 264.7 cells and mice. journal of functional foods. 2015; 13: 108-16.
- Zhang Y, Huang R, Cheng M, Wang L, Chao J, Li J, et al. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome. 2019; 7(1): 1-16.
- Yao X, Zhang C, Xing Y, Xue G, Zhang Q, Pan F, et al. Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nature communications. 2017; 8(1): 1-17.
- Pierantonelli I, Rychlicki C, Agostinelli L, Giordano DM, Gaggini M, Fraumene C, et al. Lack of NLRP3-inflammasome leads to gut-liver axis derangement, gut dysbiosis and a worsened phenotype in a mouse model of NAFLD. Scientific reports. 2017; 7(1): 1-15.

