Case Report - Volume 3 - Issue 1
Imaging findings of mitral arcade
Diala Steitieh MD1,*; Mostafa Naguib MD2; Harsimran Singh MD1; Su Yuan MD1
1Division of Cardiology, Department of Medicine, Weill Cornell Medical College, New York Presbyterian Hospital, New York, NY.
2Department of Medicine, Morristown Medical Center, Morristown, New Jersey.
Received Date : Dec 08, 2022
Accepted Date : Jan 13, 2023
Published Date: Feb 04, 2023
Copyright:© Diala Steitieh 2023
*Corresponding Author : Diala Steitieh, Division of Cardiology, Department of Medicine, Weill Cornell Medical College, NewYork-Presbyterian Hospital, New York, USA.
Email: dis2012@nyp.org
DOI: Doi.org/10.55920/2771-019X/1365
Introduction
Mitral regurgitation (MR) is the most common valvular abnormality in adults. Mitral regurgitation affects nearly 2% of the total population and nearly 10% of those age ≥75 years [1]. While the most prevalent etiology of primary MR is mitral valve prolapse (MVP) [2], rarer causes include anomalous mitral arcade (MA), also referred to as mitral hammock. Anomalous MA is a rare congenital malformation of the subvalvular apparatus of the mitral valve, characterized by absent or shortened chordae tendineae with thickened and elongated papillary muscles and a fibrous tissue band connecting the anterolateral and posteromedial papillary muscles and/or the free edge of the anterior mitral leaflet [3–5]. This fibrous band between the two pillars of the papillary muscles appears like the arch of an “arcade” when viewed from the left ventricle [3–5]. Layman and Edwards first described this anomaly in 1967 in 3 children after autopsy [4]. Since then, the majority of cases of MA have been documented in the pediatric literature. In total, 18 adult cases have been reported [6–9]. We aimed to add to current literature by presenting the case of a 24-year-old woman with mitral arcade and severe mitral regurgitation who underwent successful complex valve repair
Case Presentation
A 24-year-old woman with past medical history of fibroids presented with palpitations and progressive resting dyspnea. On examination, she was in decompensated heart failure with lower extremity edema and bibasilar crackles. ECG showed new onset atrial fibrillation that successfully converted to sinus rhythm after treatment with diltiazem. A transthoracic echocardiogram (TTE) showed normal left ventricular systolic function but severe mitral regurgitation with severe left atrial dilatation, and elongated papillary muscles with short chordae [Figure 1]. The parasternal long axis view showed doming of the anterior mitral valve leaflet [Figure 2]. A diagnosis of rheumatic valve disease was less likely however due to the lack of commissural fusion or calcification.
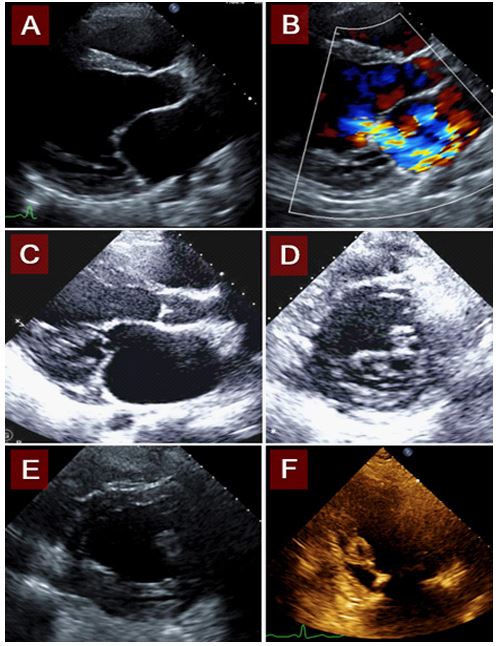
Figure 1: A.TTE shows severely dilated left atrium (with an LA volume index of 97.3ml/m2). B. Color doppler revealed severe mitral regurgitation, with a large regurgitant jet that extends to the superior left atrial wall. C. Elongated papillary muscles, short chordae inserting near leaflet tips. D. Fibrous material below the mitral valve, between papillary muscles. E. Connected papillary muscles. F. Elongated papillary muscles with short chordae.
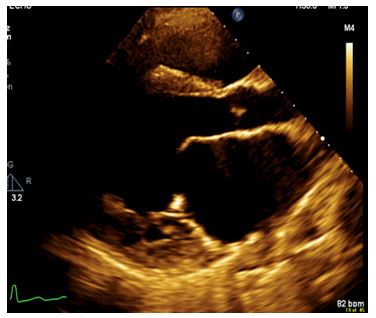
Figure 2: TTE exhibits doming of the anterior leaflet of the mitral valve, and long papillary muscles with short chordae to the posterior leaflet. Doming of the leaflets during diastole is often observed in mitral arcade.
The patient underwent transesophageal echo (TEE) to further assess the etiology of her MR. On TEE, the mitral valve leaflets failed to coapt and the papillary muscles were elongated with markedly shortened chordae tendineae [Figure 3].
Figure 2: Effects of microbiome changes on alteration in the methylation pattern of vitamin D-related genes during vitamin D supplementation.
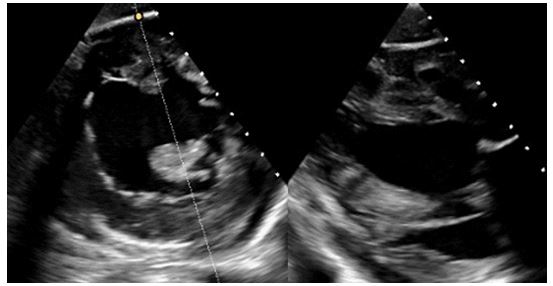
Figure 3: TEE short axis with a cross section at the level of the papillary muscles. Thickened and elongated papillary muscles attach almost directly onto the mitral valve leaflets with very short chordae tendineae.
These findings are consistent with anomalous MA. The patient underwent successful complex mitral valve repair with mitral annuloplasty, augmentation of the posterior leaflet (P1-P3) with an autologous pericardial patch, delamination of the posterior mitral leaflet and posterior papillary muscle, and insertion of a Gore-Tex neo-chordae to the P2 scallop [Figure 4]. She also underwent left atrial MAZE procedure and ligation of the left atrial appendage. Postoperative TTE showed trace residual mitral regurgitation [Figure 5]. The patient had an uncomplicated hospital course. She remained in sinus rhythm and free from heart failure.
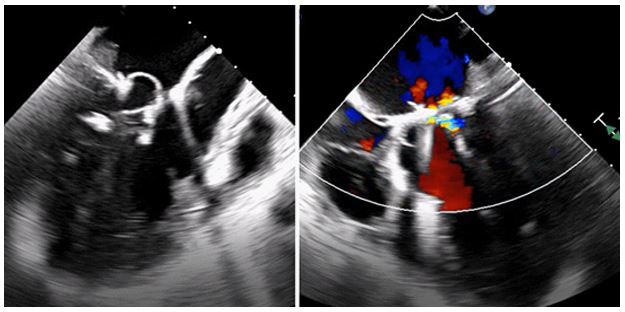
Figure 4: Intraoperative TEE. Mitral valve repair with mitral annuloplasty ring and residual trace mitral regurgitation.
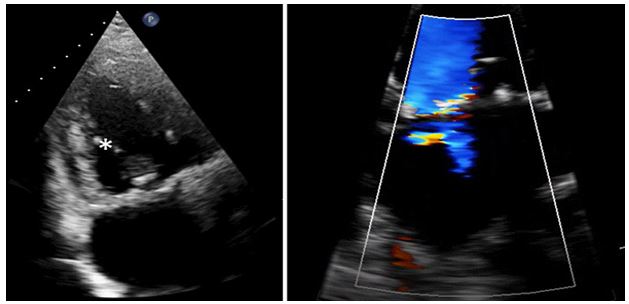
Figure 5: Post operative TTE with echodensities at the base of both mitral leaflets consistent with a mitral anuloplasty ring, and neo-chordae now attached (*). Color doppler demonstrates trace residual mitral regurgitation.
Discussion
We present an unusual case of mitral regurgitation in an adult caused by anomalous mitral arcade. Adults diagnosed with MA tend to have milder valve pathology compared to children, which explains their delay in diagnosis until adulthood [3]. Although the exact cause of MA remains unclear, it is hypothesized to be due to arrested development of the chordae tendineae apparatus during mitral valvulogenesis, notably at a stage after collagenization (conversion of muscle to fibrous tissue) but before attenuation and elongation of the tensor apparatus [4]. The premature interruption of the maturation and elongation of the tensor apparatus yields the characteristic absent or shortened chordae tendineae, and frequent direct attachment of the papillary muscles onto the valve leaflets[10,11]. This is often accompanied by a decrease in inter-chordal spaces, or their loss due to fusion of the chordae tendineae by an interconnecting fibrous band that gives rise to the classic “arcade” or “hammock” appearance of the mitral valve apparatus [3]. This malformation of the subvalvular apparatus also causes undue restraint on the interposed papillary-chordae-mitral leaflet structure, which results in improper positioning of the MV apparatus, aberrant leaflet apposition and consequent suboptimal motion of either/both leaflet(s) that leads to valve stenosis and/or regurgitation [12].
The diagnosis of mitral arcade is made through cardiac imaging. TTE is often the initial test of choice to evaluate mitral valve anatomy and pathology. In one systematic review, the diagnosis of mitral arcade was made in 50% of patients through TTE imaging alone [3].
The key defining features on TTE are: (1) Normal size of the mitral valve annulus; (2) thickened mitral leaflets with restricted motion; (3) elongated papillary muscles; (4) short or absent chordae tendinae that attach very close to or directly onto the free edge of the anterior mitral leaflet; and (5) narrow interchordal spaces and/or hammock-shaped fibrous connection between the papillary muscles [2,6]. Usually there is relatively preserved structure of the posterior leaflet apparatus but this can be affected in more severe cases of MA [12]. When assessment by TTE is suboptimal, TEE can be used to further investigate mitral valve pathology and diagnose MA. More advanced imaging such as cardiac CT or MRI is mainly used for pre-surgical planning if warranted, or to help evaluate other cardiac disease such as a concurrent cardiomyopathy. Our patient’s TTE and TEE exhibited many of these characteristic findings, which lead to her diagnosis of mitral arcade and consequent management.
The management of adult MA patients first lies in accurate diagnosis. Given the high morbidity of childhood MA, most patients are diagnosed early in life [6]. Therefore, a high index of suspicion for MA is required when evaluating the etiology of mitral regurgitation or stenosis in adult patients. Once diagnosed, symptomatic adults with significant valve regurgitation or stenosis have typically undergone valve replacement based on pediatric experience rather than valve repair. This matches with numbers from literature: Of the 18 adult cases that have been reported, 13 patients underwent valve surgery; 11/13 (85%) underwent valve replacement (3 bioprosthetic; 8 mechanical) and 2/13 (15%) underwent valve repair [6,10,11,13]. Of the other 5/18 patients: 3 were treated medically (one due to incidentally diagnosed lung cancer), 1 died from heart failure prior to intervention, and no follow up or intervention was reported for 1 patient.
Only short- to mid-term outcome data are available. Of the 13 patients who underwent valve surgery, all 13 were live at discharge; 5/13 had no further follow up; 1/13 (8%) died suddenly at home 4 months after mitral valve repair, which had been complicated by residual mitral regurgitation and severe LV dysfunction (LV ejection fraction 22%); 7/13 (54%) were well at follow up – 3/7 had unknown duration of follow up, the remaining 4/7 were followed up to 2 weeks to 1 year after surgery. Only 1/3 medically-treated patient had follow up and was well at 48 months. These numbers are to be interpreted with caution as the true numerator and denominator are unknown.
For our patient, mitral valve repair rather than replacement was chosen in order to avoid the risks associated with anticoagulation in a young, childbearing woman who also had a history of fibroids with menorrhagia. Our patient underwent successful complex mitral valve repair and is well at more than 5 months since surgery.
Conclusion
Mitral arcade remains a rare diagnosis in adulthood. When suspected, TTE and TEE are important modalities for diagnosis and differentiation from other mitral valve disease. The utility of further cardiac imaging such as cardiac CT or MRI is typically reserved for pre surgical planning or the assessment of concurrent cardiac pathology.
Acknowledgments
None
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet [Internet]. 2006; 368(9540): 1005-11. Available from: https://pubmed.ncbi.nlm.nih.gov/16980116/
- Nishimura RA, Vahanian A, Eleid MF, Mack MJ. Mitral valve disease - Current management and future challenges [Internet]. Vol. 387, The Lancet. Lancet Publishing Group; 2016; 387: 1324-34. Available from: https://pubmed.ncbi.nlm.nih.gov/27025438/
- Hakim FA, Krishnaswamy C, Mookadam F. Mitral arcade in adults -- a systematic overview. Echocardiography [Internet]. 2013; 30(3): 354-9. Available from: https://pubmed.ncbi.nlm.nih.gov/23405983/
- Layman TE, Edwards JE. Anomalous mitral arcade. A type of congenital mitral insufficiency. Circulation [Internet]. 1967; 35(2): 389-95. Available from: https://pubmed.ncbi.nlm.nih.gov/6022807/
- Perez JA, Herzberg AJ, Reimer KA, Bashore TM. Congenital mitral insufficiency secondary to anomaious mitral arcade in an adult. Am Heart J. 1987; 114(4): 894-5.
- Ong C, Brown RM, Gershon A, Trost B, Hosseini H, Pirelli L. Mitral Arcade With Anomalous Papillary Muscles: Rare Cause of Mitral Stenosis in a Young Adult. JACC Case Rep [Internet]. 2021; 3(10): 1303-9. Available from: https://doi.org/10.1016/j.jaccas.2021.06.013
- Fritz AV, Phillips SD, Landolfo KP, Martin AK. Mitral Arcade: A Rare Cause of Valvular Disease [Internet]. Vol. 13, Circulation: Cardiovascular Imaging. Lippincott Williams and Wilkins; 2020 [cited 2022 Oct 3]. Available from: https://pubmed.ncbi.nlm.nih.gov/32019345/
- Dokollari A, Cameli M, Bisleri G, Pervez MB, Kalra DK, Demosthenous M, et al. Mitral Arcades Unexpectedly Encountered During Cardiac Surgery. J Cardiothorac Vasc Anesth [Internet]. 2021; 35(3): 914-6. Available from: https://pubmed.ncbi.nlm.nih.gov/33158711/
- Vitale N, D’Errico Ramirez A, di Bari N, Acquaviva T, Milano AD. Mitral Arcade Causing Severe Stenosis in an Adult Patient. Heart Lung Circ [Internet]. 2022; 31(2): e24-6. Available from: https://pubmed.ncbi.nlm.nih.gov/34756529/
- Singh B, Srinivasa KH, Surangi MJ, Rangan K, Manjunath CN. Anomalous mitral arcade variant with accessory mitral leaflet and chordae presenting for the first time with acute decompensated heart failure in an adult. Echocardiography [Internet]. 2013; 30(7). Available from: https://pubmed.ncbi.nlm.nih.gov/23663062/
- Adil A, Samad F, Bush ML, Galazka PZ, Tajik AJ. Familial Mitral Arcade, Tricuspid Dysplasia, Left Ventricular Noncompaction and Short-Chain Acyl-CoA Reductase Deficiency. American Journal of Cardiology [Internet]. 2020; 125(4): 652-7. Available from: https://pubmed.ncbi.nlm.nih.gov/31870493/
- Pang K, Wang J, Zhang T, Wu J, Gao Y, Liang Y, et al. Undifferentiated Chordae Tendineae of the Mitral Valve: Large Cohort Study of a Rare Mitral Malformation. Front Cardiovasc Med. 2021; 27: 755.
- Matsumaru I, Hashizume K, Ariyoshi T, Izumi K, Onohara D, Nakaji S, et al. Characteristics and treatment strategies of mitral regurgitation associated with undifferentiated papillary muscle. Gen Thorac Cardiovasc Surg [Internet]. 2012; 60(7): 406-10. Available from: https://link.springer.com/article/10.1007/s11748-012-0055-x

