Case Report - Volume 3 - Issue 1
Hematogenous septic arthritis after second degree burn and rational use of antibiotics
Mehmet Ali Oktay1*; Selin Akyüz Oktay2; Demet Küçük3; Nursel Atay4
1Merzifon Karamustafa Paşa State Hospital, Department of Child Health and Diseases, Amasya, Turkey. 2Suluova State Hospital, Department of Child Health and Diseases, Amasya, Turkey.
3Ondokuz Mayıs University Faculty of Medicine, Department of Radiology, Samsun, Turkey.
4Gazi University Faculty of Medicine, Department of Pediatric Infectious Diseases, Turkey.
Received Date : Jan 25, 2023
Accepted Date : Feb 16, 2023
Published Date: Feb 18, 2023
Copyright:© Mehmet Ali Oktay 2023
*Corresponding Author : Mehmet Ali Oktay, Department of Child Health and Diseases, Amasya, Turkey.
Email: malii-71@hotmail.com
DOI: Doi.org/10.55920/2771-019X/1376
Abstract
A 51-month-old female patient, who has meningomyelocele, presented with complaints of pain, fever, and inability to move her left leg from the hip while receiving antibiotic treatment after a localized second degree burn (9%). Physical findings, radiographic findings, and aspiration fluid from the hip joint were consistent with septic arthritis (SA). Methicillin-sensitive staphylococcus aureus growth was observed in wound culture, blood culture and synovial fluid culture sent after the operation. As the clinical condition of the patient with hematogenous spread was stable, treatment with ampicillin-sulbacsite was started. In burn patients, SA should be suspected in cases with fever and joint movement limitation, and it should be predicted that infections related to the burn may be pathogenic for SA. A rational antibiotic approach should be adopted in order to prevent resistance. Cases occurring after the development of large surface burns are available in the literature, and our case is the first pediatric case to develop septic arthritis after small surface area burn.
Keywords: Septic arthritis, Burn
Introduction
The incidence of septic arthritis (SA) in children is 1-5 per 100,000 in developed countries [1]. SA is typically monoarticular, most commonly involved in the hip and knee joints, and its prevalence is high at the ages ≤5 [1, 2]. SA can occur by three different mechanisms: the spread of the pathogen through the bloodstream, the spread through the neighborhood, or the penetration of the infectious agent. The first mechanism is the most frequently described mechanism in children [2]. While Kingella kingae causes 88% of osteomyelitis or septic arthritis cases in 6-48 months old children, Staphylococcus aureus is often the cause in older children [3]. The majority of childhood burns occur in children younger than 5 years old. Scalds are the most common cause in this age group. Burn wound infections are a serious problem as they delay epidermal maturation and lead to additional scar tissue formation. Invasion of microorganisms into the tissue layers below the dermis can also result in bacteremia, sepsis, and multiple organ dysfunction syndrome. The most common causative microorganisms are Staphylococcus aureus and Pseudomonas aeruginosa [4]. Here, we present a case of septic arthritis of the hip that developed after a second degree burn.
Case
A 51-month-old female patient, who was followed up with a diagnosis of meningomyelocele, was admitted to the pediatrics outpatient clinic with a fever of 38 degrees and above for two days, and a second degree burn developed after she fell asleep by leaning her left leg against the heater. In the physical examination of the patient who did not have any other symptoms, there were three 2x5 cm wide hyperemic burn scars with yellow pus on the lateral region of the left leg. In the blood tests of the patient, White Blood Cell (WBC) was 15.6 h x10^3/ul, Neutrophil count (NEU) was 9.7 h x10^3/ul, C-Reactive Protein (CRP) was 20 mg/l and Methicillin-sensitive Staphylococcus aureus growth was observed in wound culture. The wound was debrided by plastic surgeon and 80 mg/kg/day oral amoxicillin-clavulanate was started. In the follow-ups, it was observed that the redness of the burn scar regressed.
The patient reapplied on the 14th day of antibiotic treatment with complaints of fever, inability to move her left leg from the hip, and pain. On physical examination, there was limited range of motion in the left hip. Laboratory examinations revealed WBC 19.3 x10^3/ul, NEU 13.6 x10^3/ul, CRP 47 mg/l, and erythrocyte sedimentation rate (ESR) 41 mm/min. The left femur appeared dislocated on direct radiography (Fıgure 1). In Magnetic Resonance (MR) imaging taken with the initial diagnosis of septic arthritis, it was noted that the femoral head was dislocated from the acetabulum and was located superiorly and anteriorly. Widespread effusion and edema were observed in the surrounding muscles and joints. No bone marrow edema was detected (Fıgure 2). Repeated assays 6 hours later showed a doubling of acute phase reactants (APR) (CRP 84 mg/l ESR 98 mm/min). The patient was operated by orthopedics with the diagnosis of SA, and seropurulent fluid was drained from the joint space. 1-2 polymorph-nucleated leukocytes and many gram (+) positive cocci were found in the fluid sent from the joint space. The patient was started on 200 mg/kg ampicillin-sulbactam. Methicillin-sensitive staphylococcus aerus was seen in the blood culture sent before antibiotics and in the culture sent from the joint space, and was sensitive to ampicillin-sulbactam. Transthoracic echocardiography was performed to exclude the diagnosis of bacterial endocarditis, which may lead to hematogenous spread of the infection to the hip joint; no abnormality was detected. After 14 days of ampicillin-sulbactam treatment was completed, CRP decreased to 3 mg/L and ESR to 71 mm/min. The patient was discharged with high-dose amoxicillin-clavunate therapy. Oral treatment was continued for another 3 weeks until the sedimentation returned to the normal range. The patient received a total of 5 weeks of antibiotic therapy. Complete recovery was seen in the control MR imaging. SA, one of the rare complications associated with burns, was encountered in this case and was treated with ampicillin-sulbactam; we wanted to share this extraordinary case.
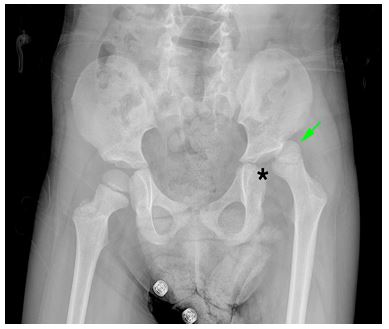
Figure 1: Hip AP X-ray shows that the femoral head (arrow) is dislocated to the superposterior of the acetabular fossa (asterisk) on the left.
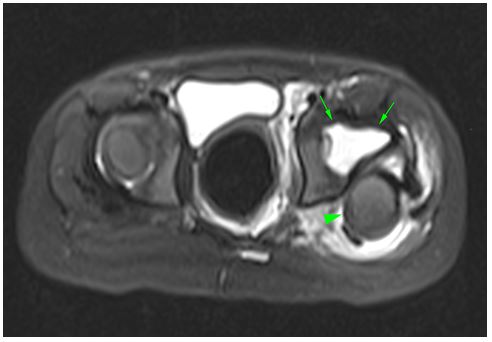
Figure 2: Axial fat-suppressed T2-weighted MRI shows a superposterior dislocation of the left femoral head (arrowhead) and a marked increase in fluid (arrows) in the joint.
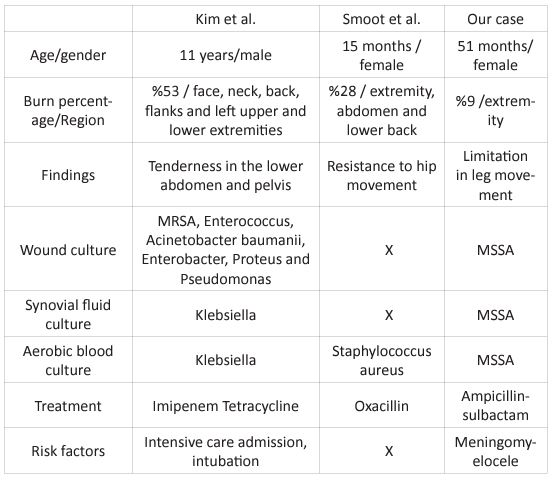
Table 1: Comparison of cases with septic arthritis after burns in the literature.
Discussion
SA is an infection with an increasing frequency in pediatric patients. SA should be carefully evaluated, diagnosed and treated to avoid devastating sequelae [2]. There are few reported cases of septic hip arthritis in burn patients [5, 6]. These cases are the cases after the development of large surface burns, and our case is the first case to develop septic arthritis after small surface area burns.
The hip is the most common site of septic arthritis in children [6]. The pathophysiology of septic arthritis of the hip is related to whether it occurs hematogenously or directly, and whether there is concomitant immunosuppression or bone-joint disease. Burn creates a relative immunosuppression state in patients [5, 6]. Blood flow in metaphyseal plaques tends to be slowed in small terminal vessels. This dynamic allows bacteria to accumulate in areas where there are few reticuloendothelial cells to fight them. Thus, it provides the infrastructure for the formation of hematogenous disseminated osteomyelitis in the end regions of the long bones; osteomyelitis in these regions can also progress to SA [5].
A laboratory panel, including CRP, ESR, blood cultures, complete blood count, should be requested in any patient with suspected SA. It has been shown that the sensitivity of ESR and CRP elevation is 98% for diagnosing acute bone and joint infections. In these cases, only 20% to 40% growth was detected in blood cultures [1]. It is also important to consider the acute time frame in which CRP responds to inflammation. A high CRP can be detected in the first 6-8 hours of infection, with a doubling time of 8 hours. Therefore, if no increase in CRP is noted 6-8 hours after the first measurement, the risk of SA is low. CRP levels may initially increase after surgery, but are expected to decrease by approximately 50% after 1 or 2 days after satisfactory irrigation and debridement. ESR level normalizes 2 to 3 weeks after an inflammatory event, while CRP should return to normal within 3 to 8 days [1]. In our case, it was observed that the CRP value obtained 6 hours after the first laboratory results doubled and returned to normal in a short time after the operation.
In SA, it is accepted that initial antibiotic therapy should be started empirically to cover pathogens potentially responsible for these infections by age group. In cases with suspected methicillin-resistant Staphylococcus aureus or post-burn Pseudomonas aeruginosa, broad-spectrum empirical antibiotic therapy is recommended [2, 4, 5]. However, there is no consensus on which molecule(s) should be used in various countries. This lack of consensus is mostly due to different antibiotic-resistant microorganisms in different parts of the world. Antibiotic selection should be individualized to include microbial profiles of different regions, taking into account the patient's age and clinical condition [2]. Although our case has a history of burn wound, it has the feature of being treated by responding to ampicillin-sulbactam, which has a relatively narrow spectrum.
SA requires rapid anti-infective therapy, starting with intravenous antibiotics and then switching to oral antibiotics to prevent complications. However, there is no consensus on the duration of antibiotics and when to switch to oral therapy. In general, it is recommended to switch to oral therapy after several weeks of intravenous antibiotic therapy, when healing is nearly achieved [2].
As a result, in burn patients with fever and joint movement limitation, the initial diagnosis of SA should be considered and it should be predicted that infections due to burns may be pathogenic for SA. In the selection of antibiotics, a rational antibiotic approach should be adopted in order to prevent the development of resistance.
References
- Erkilinc M, Gilmore A, Weber M, Mistovich RJ. Current concepts in pediatric septic arthritis. JAAOS-Journal of the American Academy of Orthopaedic Surgeons. 2020;10: 5435.
- Castellazzi L, Mantero M, Esposito S. Update on the management of pediatric acute osteomyelitis and septic arthritis. International journal of molecular sciences. 2016; 17(6): 855.
- Yagupsky P. Changing aetiology of paediatric septic arthritis. Journal of Paediatrics and Child Health. 2021; 57(10): 1560-3.
- Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clinical microbiology reviews. 2006; 19(2): 403-34.
- Kim A, Palmieri TL, Greenhalgh DG, O'Mara MS. Septic hip presenting with dislocation as a source of occult infection in a burn patient. Journal of burn care & research. 2006; 27(5): 749-52.
- Smoot EC, Graham DR, Fisk JR, Kucan JO. Development of septic arthritis by hematogenous seeding in a pediatric patient with burns. The Journal of burn care & rehabilitation. 1993; 14(1): 55-7.

