Research Article - Volume 3 - Issue 2
Dysregulation of Expression of the FOXO3 Transcription Factors Pathway in Helicobacter pylori-Associated Gastric Cancer
Lucas Matheus Vieira Da Silva1; Bruno Mari Fredi1; Laine Andreotti De Almeida1; Jessica Nunes Pereira1; Mônica Pezenatto Dos Santos1; Roger Willian De Labio1; Michelly Cristina Montenote1; Mônica Santiago Barbosa2; Marilia De Arruda Cardoso Smith3; Spencer Luíz Marques Payão1; Lucas Trevizani Rasmussen1,4*
1Faculdade de Medicina de Marília (FAMEMA), Marília, São Paulo, Brazil.
2Universidade Federal de Goiás (UFG), Goiânia, Goiás, Brazil.
3Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil.
4Centro Universitário das Faculdades Integradas de Ourinhos (UniFio), Ourinhos, São Paulo, Brazil.
Received Date : Feb 02, 2023
Accepted Date : Feb 27, 2023
Published Date: Mar 01, 2023
Copyright:© Lucas Trevizani Rasmussen 2023
*Corresponding Author : Lucas Trevizani Rasmussen, PhD, Genetics laboratory, Address: Lourival Freire, 240, Bairro Fragata, CEP 17519-050, Marília, São Paulo, Brazil.
Email: lucasrasmussen@gmail.com
DOI: Doi.org/10.55920/2771-019X/1385
Abstract
The transcription factor FOXO3 acts as a tumor suppressor and is the major target of AKT kinases pathway. The protein encoded by FOXO3, prevents the activation of genes that are important for proliferation, differentiation and death cell. In addition, FOXO3 interacts with BCL-2 family proteins; and among them, BBC3 (PUMA) and BCL2L (BIM) are responsible for regulating apoptosis through the BAX and BAK chains. This study investigated the expression of FOXO3a, BBC3 and BCL2L in gastric cancer considering the presence of Helicobacter pylori and its involvement in the modulation of gene and protein expression. A total of 225 gastric biopsy samples were evaluated from individuals with normal gastric mucosa (n=54), gastritis (n=108), and gastric cancer (n=63), of both genders and over 18 years old. Samples were subclassified according to the presence or absence of Helicobacter pylori, detected by PCR. Real-time quantitative RT-PCR (qRT-PCR) and western blotting, were used to detect the expression. FOXO3 showed increased gene (p<0.0001) and protein (p=0.036) expression in patients with gastric cancer, who also exhibited a boost in mRNA expression through H. pylori infection. BCL2L had a subtle increase in its expression independent of the presence of H. pylori (p<0.0001), while the BBC3 gene showed no alterations. Our results suggested that increase FOXO3 expression may play an important role in carcinogenesis by performing positive feedback in an attempt to eliminate neoplastic cells. FOXO3 could be suggested a prognostic marker as well as a potential molecular therapy target for gastric cancer patients.
Keywords: Apoptosis, BBC3, BCL2L, FOXO3, Gastric Cancer,
Introduction
Gastric cancer has become one of the most common neoplasms in the world. In Brazil, it is the third most frequent type of cancer among men and the fifth among women with approximately 13,360 new cases in men and 7,870 in women in 2020 [1]. Gastric cancer is considered a multifactorial neoplasm and its development depends on the participation of different factors, which may be genetic and/or environmental. Numerous studies have sought to explain the development/etiology of gastric cancer and suggested a series of genetic and epigenetic alterations, mainly in tumor suppressor genes [2,3,4].
One of the factors that can precede gastric cancer is atrophic gastritis, usually caused by Helicobacter pylori (H. pylori) infection. It is believed that half of the world population is already infected by this bacterium; however, only 15% of those infected have any symptoms or complications [23, 24]. In 1994, H. pylori was recognized as a Type I carcinogen by the WHO, and its chronic infection is the best-known risk factor for gastric cancer [5].
Considering the genetic components associated with the etiology of gastric cancer, the genes of the Forkhead box family stand out, as they are responsible for encoding proteins that control the transcription of several genes that act in the regulation of numerous biological processes. The processes include apoptosis, DNA repair, cell cycle regulation, longevity, aging, cancer, and neurogenesis, all of which are necessary to maintain homeostasis and development [6].
Of the genes present in the FOX family, researchers have reported a possible relationship between gastric cancer and the Forkhead box O3 (FOXO3) gene. FOXO3 is a tumor suppressor gene mapped to chromosome 6 (q21), which has three exons and two introns, and an intron is located within the coding sequence of the forkhead domain [7-10]. The transcription factor FOXO3 acts as a tumor suppressor and is the major target of Serine/Threonine kinase (AKT). Some genes are directly affected by the action of the FOXO3 protein, including p130(RB2), cyclin D, BBC 3, and BCL2L11, Fas ligand, and Bcl-XL. The genes of the Bcl-2 family (Bcl-XL, BCL2L11, and BBC3) are responsible for managing apoptosis through pro-apoptotic proteins, such as PUMA, BIM, and BAX/BAK [7-10].
PUMA (p53 upregulated modulator of apoptosis) is a pro-apoptosis protein mainly regulated by the tumor suppressor p53. It rapidly induces apoptosis through a BAX and mitochondria-dependent pathway [10]. One of the functions of the PUMA protein is to repair apoptosis failures in neoplastic cells, by adjusting several apoptotic pathways through interaction with some anti-apoptotic members of the BCL-2 family, which are indispensable for apoptosis induced by exogenous and endogenous p53 [12].
BIM is a pro-apoptosis protein of the BCL-2 family, encoded by the BCL2L11 gene and located in the outer membrane of mitochondria. This protein acts as an important apoptosis inducer, mediating the translocation of the apoptosis-inducing factor and mitochondrial depolarization [13,14]. The expression of this gene can be induced by the nerve growth factor as well as by FOXO3. In addition to favoring apoptosis, like PUMA, BIM works as a double agent and can promote autophagy [15].
Thus, the objective of the present work was to analyze the expression of the FOXO3, BCL2L11, and BBC3 genes and to evaluate their correlation with the gastric carcinogenesis process.
Material and Methods
Patients and Samples
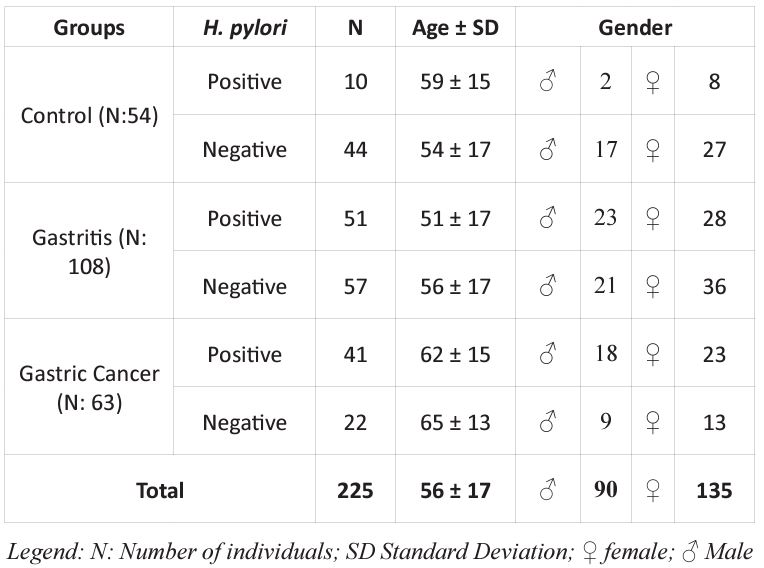
A total of 225 gastric biopsy samples from individuals of both genders and over 18 years old who had not received antiparasitic or antibiotic treatments in the last 30 days were evaluated. After collection, the samples were separated into groups: Control (patients with healthy gastric mucosa), Gastritis, and Cancer, according to histopathological analysis, following the criteria of the updated Sydney System and Lauren System. Subsequently, the groups were also divided according to the presence or absence of Helicobacter pylori (Table 1).
Table 1: Frequency of Helicobacter pylori infection, subjects and group information.
For molecular analysis, at the time of collection, the samples were stored in 2 mL tubes with RNALatter solution to ensure the integrity of the material. Then they were identified and stored in a -20 °C freezer. For protein analysis, after collection, the samples were immediately submerged in liquid nitrogen and stored at -80 °C until manipulation.
Gastric biopsy samples were collected at the Gastroenterology services of the Hospital Estadual de Bauru (HEB), the Hospital das Clínicas de Marília, and the Santa Casa de Marília. Samples from individuals with gastric cancer were obtained in collaboration with the Federal University of São Paulo (UNIFESP) and the Federal University of Goiás (UFG). This study was approved by the Research Ethics Committee of the Centro Universitário do Sagrado Coração (UNISAGRADO), Bauru, SP; under opinion number: 1,119,830.
DNA Extraction and Helicobacter pylori Detection
DNA extraction from the biopsies of the antrum region of the stomach was performed according to the protocol established by the QiAmp® DNA Mini Kit from QIAGEN (Cat No. 51304).
- pylori was diagnosed using the Polymerase Chain Reaction (PCR) technique. The pair of oligonucleotides Hpx1 [(CTGGAGARACTAAGYCCTCC (R = purine; Y = pyrimidine)] and Hpx2 (GAGGAATACTCATTGCGAAGGCGA) was used, under conditions of 40 cycles: 94C°,1 min; 59°C, 1 min, and 72°C, 1 min. After PCR, the detection of H. pylori was verified by electrophoresis, through which a fragment of 150 bp was visualized by agarose gel electrophoresis 2.5%, stained with ethidium bromide, and viewed and photographed in a transilluminator on the Imager 2200 image capture system (Innotech Corporation)
RNA Extraction, cDNA Synthesis and Real-Time Quantitative PCR (qPCR) Gene Expression Analysis
To extract the RNAs, the tissue was first processed in a lysis solution using the Precellys 24 equipment. Subsequently, the total RNA was extracted using the miRNeasy® Mini Kit (QIAGEN - Cat No. 217004). The procedures were performed following the manufacturer's instructions. The RNA concentration of each sample and the absorbance ratio (A260/A280) were measured in the NanoDrop 2000 equipment (Spectrophotometer ND – 2000 – NANODROP, USA), and only samples with a ratio value between 1.85 and 2.2 were used.
The synthesis of complementary DNA (cDNA) from total RNA was performed using the kit: High-CapacitycDNA Reverse Transcription Kits, according to the protocol established by the manufacturer.
The qPCR was performed in the ABI Prism 7500 Fast Sequence Detection System equipment, using TaqMan gene expression assay and specific probes. The relative quantification of expression was calculated using the 2-ΔΔ Ct method according to Livak and Schmittgen (2001). To analyze the mRNA expression, the following assays were used: FOXO3a (Hs00818121_m1), BCL2L11 (Hs00708019_s1), and BBC3 (Hs00248075_m1). The TBP gene (Hs00187332_m1) was used as an endogenous control (Applied Biosystems).
Protein expression analyzed by Western Blotting
Gastric biopsy fragments from the three groups analyzed (Control, Gastritis, and Cancer) were homogenized in 300μl of RIPA buffer containing inhibitors (NaF 1M, Complete Protease Inhibitor Cocktail – Roche Diagnostics, and PMSF 0.1M). Subsequently, the homogenate was centrifuged at 14.000rpm for 15min at 4°C. After centrifugation, the supernatant was transferred to new tubes. The total proteins extracted was quantified by the Bradford method, using the NanoDrop 2000 spectrophotometer (Thermo Scientific®).
The proteins were diluted in RIPA buffer with inhibitors for homogenization and stored at -80 °C until use. To perform the immunoblot, the proteins were normalized to 25 ug, separated in a 10% SDS-polyacrylamide gel, and transferred to a nitrocellulose membrane employing electroblotting. Then, the membranes were stained with ponceau, washed with 1X PBST Buffer Solution, and incubated in 5% skim milk/PBST for two hours at room temperature to block nonspecific staining. After incubation, the membranes were washed again for three minutes, twice in 1X PBST, and incubated at 4 °C overnight in the primary antibodies: anti-FOXO3a (Ab10962a) and anti-α-tubulin (Ab7291), diluted in BSA (albumin bovine serum) 5%/PBST.
Subsequently, the membranes were washed for five min, six times, in 1X PBST and incubated in the anti-mouse HRP Rabbit secondary antibodies (Abcam: 616520) on the membrane labeled with anti-α-tubulin and HRP Goat anti-rabbit IGG (Santa Cruz Biotechnology: 2004) on the membrane labeled with anti-FOXO3a, diluted in 5% BSA/PBST, for 90 min at room temperature. Finally, the membranes were washed for five min, six times, in 1X PBST and revealed using photographic films
Statistical Analysis
Data were first analyzed using the graphical box-plot method to detect outliers. When necessary, the distribution was tested using the D'Agostino & Pearson tests. For expression and association analysis, the Wilcoxon Signed Rank, Kruskal-Wallis, Brown-Forsythe, Fisher's exact, and Chi-square tests were used, depending on the groups analyzed. Values of p<0.05 were considered significant. The analyses were performed using GraphPad Prism 5 (USA).
Results
Helicobacter Pylori Detection
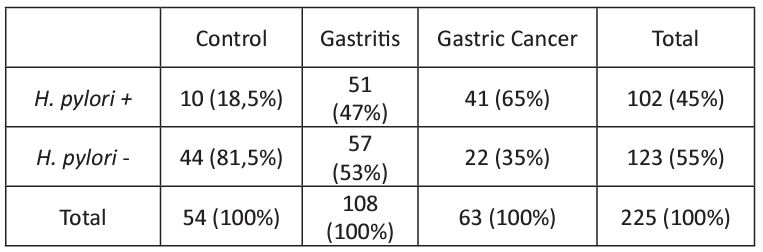
H. Pylori was detected in 102/225 (45%) gastric biopsy samples analyzed. The results indicated a higher incidence of H. pylori in the Gastritis and Cancer groups than the Control group (Table 2). The analysis revealed a significant association between the H. pylori and the development of chronic gastritis and gastric cancer. Were founded a p=0.0005; OR=3.937; CI=1.808–8.296 when comparing the Control to the Gastritis group, showing an approximately fourfold increase in the risk of developing gastritis in patients with the presence of H. pylori. When comparing the Control vs Cancer group, the result p<0.0001; OR=8,200; CI=3.442–19.800 was obtained, showing again that H. pylori increased the risk of developing gastric cancer. When comparing the Gastritis vs Cancer group, the result was p<0.0001; OR=2.083; CI=1.118–3.850.
Table 2: Frequency of H. pylori infection in the patient groups.
Analysis of Gene Expression
The results were analyzed in two stages. First, we analyzed the values obtained between the Control, Gastritis, and Cancer groups, without considering the presence of H. pylori. Subsequently, the groups were divided into subgroups considering the presence of H. pylori and again tested statistically, always being compared to the Negative Control group.
FOXO3 Gene Expression
Disregarding the presence of H. pylori, a statistically significant difference was found when comparing the three groups (Control vs Gastritis vs Cancer) (p<0.0001). The analysis between the groups (post-test) revealed a statistically significant difference in the Cancer vs Control and Cancer vs Gastritis groups, both with p<0.0001, evidencing an increase in the expression of the FOXO3 (Figure 1).
Considering the presence of the bacterium, results similar to those mentioned above were found. There was a statistically significant difference between all groups (p<0.0001). The Positive Cancer group had increased expression when compared to all other groups, a result that may suggest the influence of H. pylori on the modulation of FOXO3 expression. The aforementioned increase in expression was not statistically significant when the Cancer Positive group was compared to the Cancer Negative group, suggesting that neoplastic transformation also influences the increase in FOXO3 gene expression regardless of the presence of H. pylori. However, the results obtained in the present study indicate that the presence of bacteria associated with neoplastic transformation can potentiate the expression of FOXO3 (Figure 1).
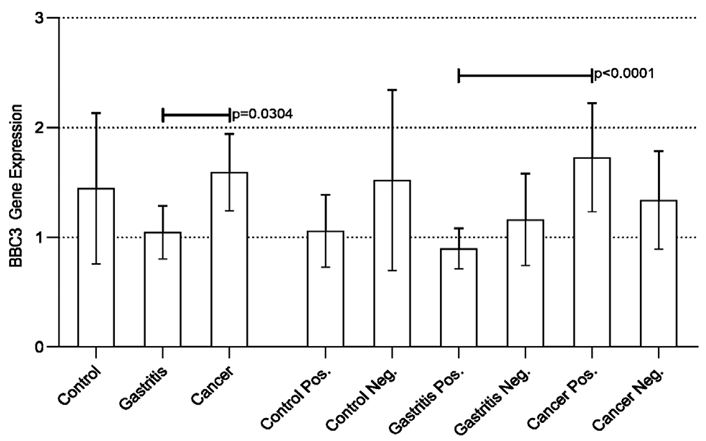
Figure 1: Real-time quantitative RT-PCR analysis of FOXO3 expression in all groups studied.
BCL2L11 Gene Expression
Following the same statistical procedure, we verified a statistically significant difference in the expression of the BCL2L11 gene, when comparing the groups (Control, Gastritis, and Cancer). A subsequent analyzes between pairs (post-test) found a statistically significant increase in the expression of the gene in the Cancer group compared to the Control and Gastritis groups. When considering the presence of H. pylori, we found a difference between all groups. However, when comparing the groups between pairs, no significant difference was observed in any analyses (Figure 2).
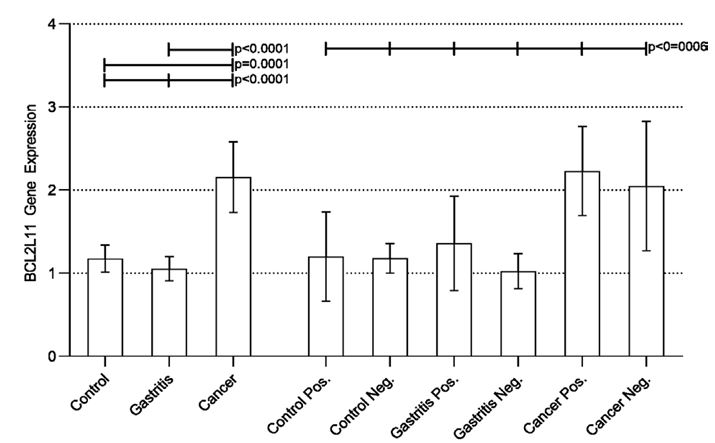
Figure 2: Real-time quantitative RT-PCR analysis of BCL2L11 expression in all groups studied.
BBC3 Gene Expression
Interestingly, the BBC3 gene showed a statistically significant difference only when comparing the Cancer vs Gastritis group (Figure 3). Considering the presence of the bacterium, only the comparison between the Positive Gastritis and Positive Cancer groups was statistically significant (Figure 3).
Protein Expression Analysis
For protein analysis, the same statistical treatment was performed, initially disregarding the presence of H. pylori with later analysis considering the presence of the bacterium.
The protein expression of FOXO3 did not have statistically significant differences in any analysis considering the Gastritis group. The results were p=0.0601 when comparing the Normal Control group vs. Gastritis and ep=0.1529 and ep= 0.0970, when analyzing the Normal vs. Control group and Positive Gastritis and Normal Control vs. Negative gastritis, respectively.
When comparing the Normal Control group vs. Cancer, without taking into account the presence of H. pylori, the test resulted in p=0.0360, demonstrating an increased expression of FOXO3 protein in patients with gastric cancer. When comparing Normal Control with the Cancer Positive and Cancer Negative groups, we found statistically significant results, with p=0.0001 in all tests. When comparing Gastritis vs. Cancer, the statistically significant difference was p=0.0360, also indicating an increased protein expression in patients with gastric cancer. Thus, the results show that FOXO3 independent of H. pylori is more expressed in gastric cancer tissues and does not seem to influence cases of gastritis.
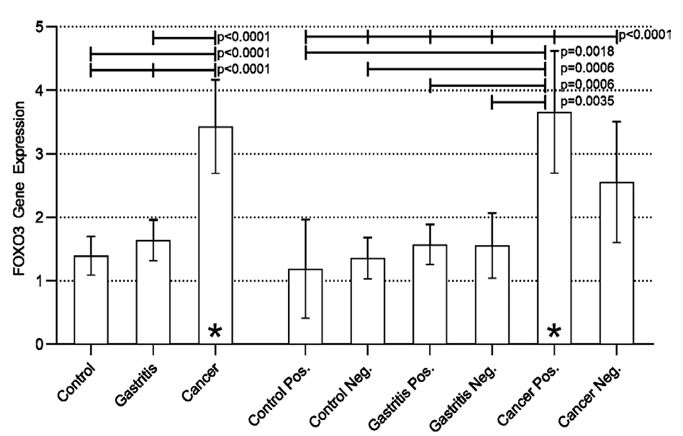
Figure 3: Real-time quantitative RT-PCR analysis of BBC3 expression in all groups studied.
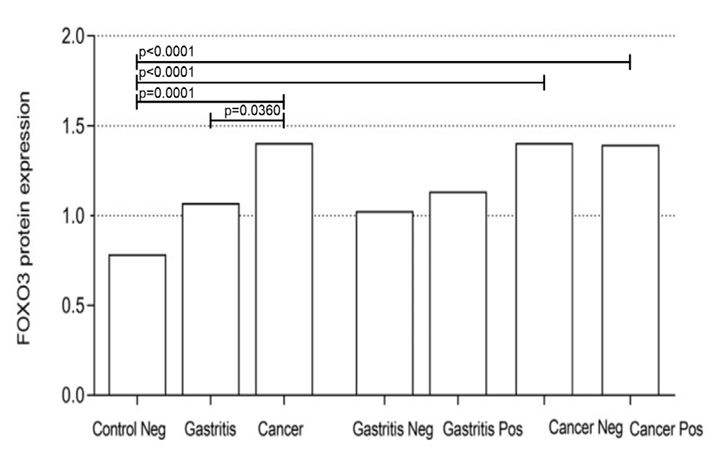
Figure 4: Protein expression of FOXO3 as evaluated by western blotting. (A) Representative result of FOXO3 protein expression (C+: Positive Cancer; C-: Negative Cancer; G+: Positive Gastritis; G-: Negative Gastritis; NC: Normal Control, H. pylori negative; TUB: Tubulin.). (B) Relative FOXO3 protein expression levels was remarkably increase in gastric tumor tissues compared with other groups.
Discussion
Several researchers have studied the bacterium H. pylori and its association with gastric diseases since the studies initiated by Marshall and Warren in 1983, with a worldwide consensus that H. pylori infection influences the development of pathologies that mainly affect the gastric tissue. [16] demonstrated a significant difference in the risk of developing gastric diseases with the presence of H. pylori, which agrees with the present study [16].
Recently [16], reported that FOXO3 is downregulated in gastric cancer tissues, a result that agrees with the results obtained in the present study. Note that Li M. et al. used gastric paracarcinoma as a control, which may have directly influenced the results obtained [17]. On the other hand [18], conducted a study involving cells infected and not infected by H. pylori and suggested that the bacterium acts in the phosphorylation of the FOXO3 protein, leading to its inactivation and translocation from the nucleus to the cytoplasm. As it is a pro-apoptosis protein, when inactivated, FOXO3 ceases to exert the transcriptional activity of pro-apoptotic genes and manage the cell cycle, favoring tumor progression. The increased expression seen in the present study could be a response mechanism to the inactivation of the FOXO3 protein in patients who are failing in the apoptosis pathway; thus, the increase in FOXO3 mRNA could increase protein production in an attempt to compensate for its poor performance [17]. Hu et al., 2015 reported in their research that FOXO3 may serve as a prognostic marker and therapeutic target in gastric cancer. Furthermore, its activation reverses the phenotype of cancer cells, and protein silencing increases cancer cell motility [17].
The results obtained by Li et al. (2020) indicate that the FOXO3 protein is downregulated in carcinogenic tissues. However, the study used gastric paracarcinoma material from the same patients as a control, thus, FOXO3 would be more expressed at the edge of the tumor than at the center. The current study compared carcinogenic tissues with tissues from a healthy mucosa [17].
The results of the present study indicate that greater expression of FOXO3 could be a positive feedback mechanism attempting to reverse the neoplasm picture, as already suggested in the analysis of gene expression.
For mRNA expression of BCL2L11, the results agree with Zhang H, Duan J, Qu Y et al. 2016, who found no statistical difference in patients with gastric cancer [15]. When evaluating if H. pylori has an influence, Akazawa and colleagues evaluated in 2015 the expression of the BCL2L11 gene, and their results showed a significant increase in the expression of BCL2L11 in patients with the presence of H. pylori, noting that the degree of gastritis directly affects gene expression [20]. The results were partly replicated in the present study.
In a more recent analysis, in 2021, Mu J and collaborators performed an experiment using animals. Their results confirmed that PUMA is an apoptosis regulator, and its expression influences tumor progression [22]. A year earlier, in 2020, Yini Dang correlated the expression of BBC3 with the bacterium H. pylori, in gastritis samples. The resulting expression was four-times higher in the presence of the bacterium, and in the cell culture of cancer tissue, the expression was elevated in the presence of H. pylori [22].
Hu et al., 2015 reported in their research that FOXO3 may serve as a prognostic marker and therapeutic target in gastric cancer. Furthermore, its activation reverses the phenotype of cancer cells, and protein silencing increases cancer cell motility [19].
Li, et al., 2020 obtained results that indicate that the FOXO3 protein is downregulated in carcinogenic tissues. However, the study used gastric paracarcinoma material from the same patients as a control, thus, FOXO3 would be more expressed at the edge of the tumor than at the center. The current study compared carcinogenic tissues with tissues from a healthy mucosa [17].
Conclusion
The results obtained about the expression of FOXO3 mRNA and protein suggest that it could be resulting in positive feedback in response to changes caused by neoplasia. Furthermore, this work demonstrated that Helicobacter pylori plays an important role not only in the development of gastric cancer but also in boosting FOXO3 mRNA expression.
BCL2L11(BIM) and BBC3 (PUMA) do not seem to have significant effects on their expression due to the cascade of events triggered by FOXO3, H. pylori, and neoplasm. However, these findings may be related to the dual role of these molecules in both autophagy and cellular apoptosis.
Acknowledgments: The authors are grateful for the financial support of the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), grant numbers: 2018/08481-1 and 2018/02008-2 and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Code: 001)
Conflicts of Interest: No conflicts of interest to declare.
Data Availability Statement: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- INCA (Instituto nacional de câncer) IINd. Câncer de Estômago [Available from: https://www.inca.gov.br/tipos-de-cancer/cancer-de-estomago/profissional-de-saude.
- Liu X, Meltzer SJ. Gastric Cancer in the Era of Precision Medicine. Cell Mol Gastroenterol Hepatol. 2017; 3(3): 348-58.
- Necula L, Matei L, Dragu D, Neagu AI, Mambet C, Nedeianu S, et al. Recent advances in gastric cancer early diagnosis. World J Gastroenterol. 2019; 25(17): 2029-44.
- Tamura G. Alterations of tumor suppressor and tumor-related genes in the development and progression of gastric cancer. World J Gastroenterol. 2006; 12(2): 192-8.
- Kodaira MS, Escobar AM, Grisi S. [Epidemiological aspects of Helicobacter pylori infection in childhood and adolescence]. Rev Saude Publica. 2002; 36(3): 356-69.
- DaSilva L, Kirken RA, Taub DD, Evans GA, Duhé RJ, Bailey MA, et al. Molecular cloning of FKHRL1P2, a member of the developmentally regulated fork head domain transcription factor family. Gene. 1998;221(1):135-42.
- Butt AM, Feng D, Idrees M, Tong Y, Lu J. Computational identification and modeling of crosstalk between phosphorylation, O-β-glycosylation and methylation of FoxO3 and implications for cancer therapeutics. Int J Mol Sci. 2012; 13(3): 2918-38.
- Flachsbart F, Möller M, Däumer C, Gentschew L, Kleindorp R, Krawczak M, et al. Genetic investigation of FOXO3A requires special attention due to sequence homology with FOXO3B. Eur J Hum Genet. 2013; 21(2): 240-2.
- Li M, Zhang H, Wang JX, Pan Y. A new essential protein discovery method based on the integration of protein-protein interaction and gene expression data. BMC Syst Biol. 2012; 6: 15.
- Tikhanovich I, Kuravi S, Campbell RV, Kharbanda KK, Artigues A, Villar MT, et al. Regulation of FOXO3 by phosphorylation and methylation in hepatitis C virus infection and alcohol exposure. Hepatology. 2014; 59(1): 58-70.
- Han J, Flemington C, Houghton AB, Gu Z, Zambetti GP, Lutz RJ, et al. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci U S A. 2001; 98(20): 11318-23.
- Day CL, Smits C, Fan FC, Lee EF, Fairlie WD, Hinds MG. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol. 2008; 380(5): 958-71.
- Concannon CG, Tuffy LP, Weisová P, Bonner HP, Dávila D, Bonner C, et al. AMP kinase-mediated activation of the BH3-only protein Bim couples energy depletion to stress-induced apoptosis. J Cell Biol. 2010;189(1):83-94.
- Kilbride SM, Farrelly AM, Bonner C, Ward MW, Nyhan KC, Concannon CG, et al. AMP-activated protein kinase mediates apoptosis in response to bioenergetic stress through activation of the pro-apoptotic Bcl-2 homology domain-3-only protein BMF. J Biol Chem. 2010; 285(46): 36199-206.
- Zhang H, Duan J, Qu Y, Deng T, Liu R, Zhang L, et al. Onco-miR-24 regulates cell growth and apoptosis by targeting BCL2L11 in gastric cancer. Protein Cell. 2016; 7(2): 141-51.
- Zabaglia, L. M, Sallas, M. L, Santos, M, Orcini, W. A, Peruquetti, R. L, Constantino, D. H, Chen, E, Smith, M, Payão, S. M, Rasmussen, L. T. Expression of miRNA-146a, miRNA-155, IL-2, and TNF-α in inflammatory response to Helicobacter pylori infection associated with cancer progression. Annals of human genetics. 2018; 82(3): 135-142.
- Li M, Wang Y, Liu X, Zhang Z, Wang L, Li Y. miR-629 targets FOXO3 to promote cell apoptosis in gastric cancer. Exp Ther Med. 2020; 19(1): 294-300.
- Tabassam FH, Graham DY, Yamaoka Y. Helicobacter pylori-associated regulation of forkhead transcription factors FoxO1/3a in human gastric cells. Helicobacter. 2012; 17(3): 193-202.
- Hu C-AA, White K, Torres S, Ishak M-A, Sillerud L, Yubin, et al. Chapter 10 - Apoptosis and Autophagy : The Yin–Yang of Homeostasis in Cell Death in Cancer. 2015.
- Akazawa Y, Matsuda K, Isomoto H, Matsushima K, Kido Y, Urabe S, et al. BH3-only protein Bim is associated with the degree of Helicobacter pylori-induced gastritis and is localized to the mitochondria of inflammatory cells in the gastric mucosa. Int J Med Microbiol. 2015; 305(6): 553-62.
- Mu, J, Sun, X, Zhao, Z, Sun, H, & Sun, P. BRD9 inhibition promotes PUMA-dependent apoptosis and augments the effect of imatinib in gastrointestinal stromal tumors. Cell death & disease. 2021; 12(11): 962.
- Dang Y, Zhang Y, Xu L, Zhou X, Gu Y, Yu J, et al. PUMA-mediated epithelial cell apoptosis promotes Helicobacter pylori infection-mediated gastritis. Cell Death Dis. 2020; 11(2): 139.
- Quintans J FU, Barreto A, Federal U, Jos L, Federal U. Aspectos Gerais nas Infecções por Helicobacter Aspectos Gerais nas Infecções por Helicobacter pylori. 2007;(October 2016). 3.
- Suzuki R, Shiota S, Yamaoka Y. Molecular epidemiology, population genetics, and pathogenic role of Helicobacter pylori. Infect Genet Evol. 2012; 12(2): 203-13.

