Case Report - Volume 3 - Issue 2
Unusual presentation of Giant Dermatofibrosarcoma Protuberans
Idrissi OK1*; Hali F1; Jadib I2; Bachkira M2; Hadad O2; Messoudi A2; Mernissi F3; Chiheb S1
1Dermatology department, Ibn Rochd Hospital, Hassan II University, Morocco.
2Orthopedic and traumatology surgery department, Ibn Rochd Hospital, Hassan II university, Morocco.
3Anatomic pathology department, Ibn Rochd Hospital, Hassan II University, Morocco.
Received Date : Feb 08, 2023
Accepted Date : Feb 28, 2023
Published Date: Mar 07, 2023
Copyright:© Idrissi OK 2023
*Corresponding Author : Idrissi OK, Dermatology department, Ibn Rochd Hospital, Hassan II University, Morocco.
Email: oumayma.kidrissi@gmail.com
DOI: Doi.org/10.55920/2771-019X/1389
Abstract
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, soft tissue sarcoma with a low metastatic potential but high rate of recurrence after surgical intervention. This tumor is characterized by a long history of slow growth. DFSP usually occurs on trunk and proximal extremities but rarely touches distal extremities such as hands, fingers, or foot below knees. We present a case of giant DFSP of the distal extremity, with a post-traumatic onset in childhood and a very long course. A 45-year-old patient presented with a tumoral lesion of the foot, evolving since 35 years with an onset at the age of 10 years, after a strong trauma of the 3rd toe. Physical examination revealed a reddish purple polynodular neoplastic lesion with a firm consistency. One of these nodular masses was ulcerated with areas of necrosis. The CT scan did not detect any bone involvement. Magnetic resonance imaging revealed a heterogeneously enhancing mass lesion on the dorsal aspect of the left forefoot. In depth, this mass invaded the dorsal interosseous muscles. The histopathological study was in favor of DFSP, with evidence of spindle-shaped tumor cells arranged in a storiform pattern. On immunohistochemistry (IHC), the cells expressed intense and diffuse CD34 and were negative for Desmin and S-100. The localization or occurrence of DFSP after a long period of trauma should not mislead the diagnosis. A careful histological study with IHC should be carried out in order to set up an appropriate treatment.
Introduction
Dermatofibrosarcoma protuberans (DFSP) is an uncommon superficial soft tissue sarcoma [1], accounts for <0.1% of all the cutaneous neoplasms and 1.8 % of all soft tissue's sarcomas [2], is defined by the World Health Organization as a superficial sarcoma with slow growth, locally aggressive and with low metastatic potential [3]. DFSP was first described by Sherwell and Taylor in 1890. In 1924, F.J. Darier and M. Ferrand described it as a progressive and relapsing dermatofibroma. Also, they emphasized the evolving and recurrent nature of the tumor[4]. In 1925, Hoffman has associated the term “protuberans” with the prominent appearance of the lesion [5]. Dermatofibrosarcoma protuberans occurs most often in adults between the third and fifth decade of life, but has been reported in all age groups, including congenital presentations [6]. The onset can be correlated with a local trauma or scar, initially as papule or plaque and subsequently taking a protuberant multinodular appearance [7]. The most common sites of involvement are the trunk in 50% to 60% of cases, the proximal limbs in 25%, and the head and cervical region in 10% to 15% of cases [6]. Rarely this tumor can occur in the distal extremities and at acral sites [2,3]. This may result in non-recognition of DFSP or delayed diagnosis, resulting in misdiagnosis or delayed treatment. Due to the rarity of the presentation on the distal limbs, the giant clinical aspect and the 35 years evolution. We believe that the reporting of this case is relevant.
Case Presentation
A 45-year-old woman with no significant medical history was referred for evaluation of a 35-year-old protruding mass on the dorsal aspect of the left foot, with onset at age 10, a few months after a severe 3rd toe trauma. The mass became painful and evolved rapidly over the past 6 years. No other systemic or local symptoms were reported. Local examination revealed a red-violet polynodular neoplastic lesion measuring 80×40 mm, firm in consistency, adherent to the subcutaneous tissue, painless on the dorsal aspect of the forefoot. One of the central nodular lesions showed ulceration and necrosis (Figure 1a, 1b). The lesion evolved according to the history, in two stages, initially as an erythematous or purple plaque, slightly infiltrated, followed by a nodular mass, slowly increasing in size. There was no clinical evidence of associated regional lymphadenopathy.
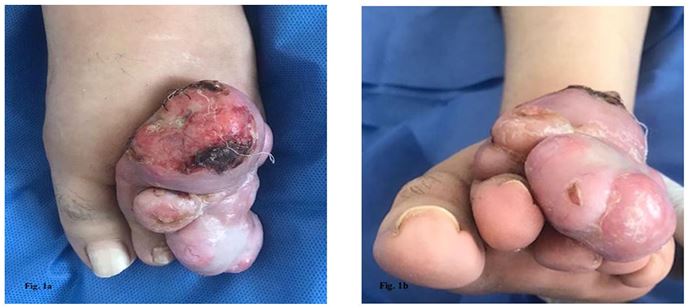
Figure 1a, 1b : Clinical morphology of multinodular violaceous lesion : DFSP
Standard radiographs showed a soft tissue mass with no bone involvement, erosion, or fracture. A CT scan was performed and revealed a suspicious heterogeneous mass that refer to a malignant lesion. No distant metastasis was found. Magnetic resonance imaging scan was suggestive of heterogeneously enhancing mass lesion (90 cm × 60 cm × 37 mm) of the dorsal side of the left forefoot. In depth, this mass invades the dorsal interosseous muscles and comes into contact with the distal part of the short and long extensor tendons of the toes. It was also associated with a diffuse infiltration of the plantar interosseous muscles. There was a discreet hypersignal from the bone marrow of the 2nd and 3rd proximal phalanges of the 3rd toe without loss of cortical hypo signal and without abnormal contrast uptake detectable post-contrast enhancement. Histopathological examination (Figure 2a, 2b, 2c) of the lesion revealed a spindle cell proliferation with a focal storiform pattern. The cells have a sparse and ill-defined cytoplasm and the nuclei are elongated with fine chromatin. mitotic activity is very low. This proliferation includes adipocytes. The immunohistochemical study shows a diffuse and intense expression of CD 34 cells. They are negative for desmin, H-caldesmon and PS 100. Ki-67 is focally positive in 8%. The findings were consistent with low-grade fusocellular mesenchymal neoplasia, favoring the diagnosis of DFSP. The case was discussed in the multidisciplinary reunion, the patient underwent a Lisfranc amputation (Figure 3). The surgical margins were tumor-free. Clinical and paraclinical monitoring will be continued because of the high risk of local relapses in the first five years after surgical excision.
Discussion
Dermatofibrosarcoma protuberans is a soft tissue tumor that usually arises in the dermis and then spreads to the subcutaneous tissues [8]. In rare cases, DFSP may occur in subcutaneous fat without dermal involvement or with minimal and clinically subtle dermal involvement. [8]. It has low metastatic potential but significant subclinical extension and high morbidity due to its local invasion and high recurrence rate [9]. The pathogenesis of DFSP remains unknow [10]. Cytogenetic and molecular studies showed that approximately 90 % of DFSPs expressed chromosomal translocation t (17;22) (q22;q13) [2]. This translocation results in the collagen 1A1 (COL1A1) gene from chromosome 17 fusing with the platelet-derived growth factor B (PDGFB) gene on chromosome 22 [11]. The product of this fusion causes dysregulation of PDGFB with subsequent activation of PDGF receptor β protein tyrosine kinase, resulting in tumorigenesis which promotes DFSP cell growth [12]. Gene fusion transcript of COL1A1− PDGFB can be detected by either fluorescent in situ hybridization (FISH) or multiplex reverse transcriptase-polymerase chain reaction (RT-PCR) in formalin fixed, paraffin embedded tissues [13]. This is valuable for the differential diagnosis and guiding treatment of DFSP.However, in 8% of DFSP cases, this fusion product cannot be detected, suggesting that other genes may be involved in this translocation [14]. In the case described above, the area of skin on which the DFSP subsequently developed suffered blunt trauma. A history of trauma in the region affected is reported in 10% to 20% as a possible etiological factor of DFSP [15]. DFSP has been reported to arise in areas with history of prior trauma, including tattoos, vaccination sites, burn scars, surgical scars and radiation [7]. The exact mechanism in which trauma may predispose for development of DFSP remains unknown, Boukovalas et al suggests that chronic inflammation and stimulation of the immune system at the site of trauma can trigger immunopathological changes that could lead to malignant transformation of dermal cells [16]. A similar mechanism has been described in the pathogenesis of Marjolin ulcers after trauma or skin injury [17]. Clinically, acral DFSPs have similar characteristics to lesions
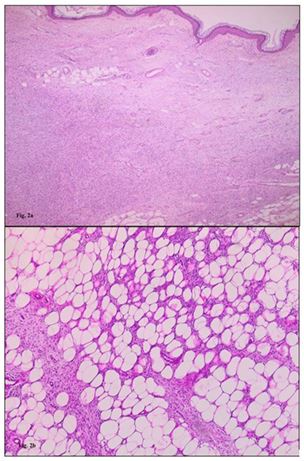
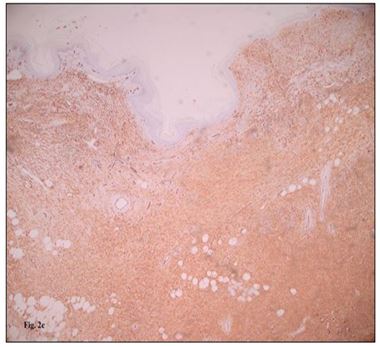
Figure 2a, 2b, 2c : Histopathologic and immunophenotypic morphology of DFSP. (A) The fibrohistiocytic proliferation. (B) The storiform arrangement of fibrohistiocytic neoplastic cells with a pauci-inflammatory background. Diffuse and strong staining with CD34 immunohistochemistry (CD34, [C]).
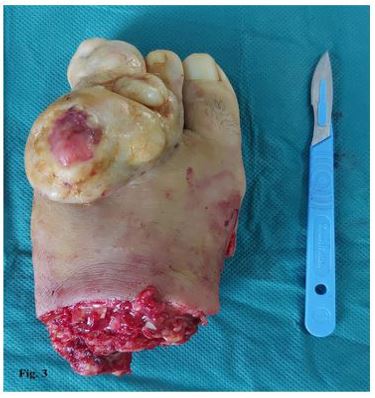
Figure 3 : Lisfranc Amputation.
at other sites. These tumors are characterized by a long history of indolent and slow growth [6]. The evolution of an indurated dermal plaque or nodule to a noticeable, symptomatic, protruding multinodular mass usually takes several years [18]. In the initial phase of radial growth, tumors are attached to the overlying skin without involvement of deeper structures [19]. Tumors may then enter a rapid growth phase where they begin to infiltrate vertically and attach to deep subcutaneous tissue structures; they may invade deeper fascia, muscle, periosteum, and bone [19]. Metastasis occurs in fewer than 5% [6,20]. Factors associated with an increased risk of metastatic disease include age greater than 50 years, increased cellularity, high mitotic index, multiple recurrences, positive microscopic margins, location in the head and neck region, and large size [9]. The evolution of the presented case corresponds to the classical variations described, with absence of metastasis despite the enormous size. It usually metastasizes to the lungs, while lymph node metastases are rare [6]. Dermatofibrosarcoma's Imaging characteristics are variable, and no specific ones were reported [2]. Computed tomography (CT) is not indicated for DFSP diagnosis. However, it is very useful in cases where underlying bone involvement is suspected or in cases of pulmonary metastases. It reveals nodular or lobular subcutaneous architecture, soft tissue attenuation, and the presence of intermediate to high contrast enhancement [1]. Magnetic resonance imaging (MRI) is very useful for the precision of tumor invasion, mainly in large tumors as in our patient. In addition, MRI can help in the differential diagnosis in cases of tumors that arise in an atypical site [15]. Conventional T1-weighted images show hypointense signal. However, it may be difficult to separate a border from fat on conventional T2-weighted images without fat saturation. Finally, enhancement can be variable and depends on the levels of necrosis or hemorrhage [21]. Histologically, acral DFSP typically has uniformly monomorphic, minimally atypical spindle cells arranged in a storiform pattern [22]. Cell nuclei are elongated with regular chromatin and low to moderate amounts of cytoplasm. Neoplastic cells often infiltrate the surrounding adipose tissue in a honeycomb pattern [22]. Mitotic activity is typically very low [3]. Immunohistochemically, DFSP tumors generally show strong and diffuse expression of CD34, but negative expression for other biomarkers, including alpha smooth muscle actin, factor XIIIa, S-100 and melanin-A [20]. Multiple histologic types of DFSP have been described [3], including myxoid, pigmented, giant cell, giant cell fibroblastoma, granular cell, sclerotic and fibrosarcomatous (FS) component. These variants reflect the morphologic heterogeneity which is associated with the spindle cell differentiation during tumor development. They do not bear significant clinical manifestations and outcomes, except for the FS variant with increased risk of local recurrence and metastatic potential [3]. The fibrosarcomatous component has greater cellularity, usually with increased mitotic activity, occasional necrosis, and a fascicular rather than storiform architecture [20], with long sweeping fascicles of spindle and sometimes forming a herringbone pattern [20]. CD34 expression is often decreased or lost in DFSP with fibrosarcomatous transformation [6]. Awareness of an atypical clinical presentation and deep biopsy are necessary to diagnose its rarer variants. The gold standard treatment for DFSP is complete surgical excision with histologically negative margins [6]. Surgical techniques include wide local excision (WLE) with tumorfree margins, Mohs micrographic surgery (MMS), partial or total amputation if the tumor is located on the upper or lower fingers [1]. Both MMS and WLE generally require wide and deep excision of the tumor with free margins of at least 3 cm from the tumor periphery [19]. In our case, it challenged us due to the giant size of the tumor and the complex invaded structures. Therefore, amputation was the preferred option to achieve tumor-free margins and allow faster return to function, but also to avoid recurrences. For adjuvant treatment, Imatinib mesylate, an inhibitor of protein tyrosine kinase, has been approved for adults in unresectable, recurrent and/or metastatic DFS that shows the t(17, 22) (q22,q13) translocation [2,12]. Radiotherapy is indicated for inoperable primary tumors, patients with positive margins when further surgery is not feasible, or as adjuvant therapy after further resection for recurrent DFSP [9,24]
Conclusion
We present this rare case of acral dermatofibrosarcoma protuberans, the singularity of the case consisted not only in the rare localization but also in the post-traumatic onset in childhood, the dramatic clinical appearance and the progressive evolution in more than 35 years. The morphological and histopathological characteristics of distal extremity DFSP are similar to those of the more common localizations, although the local anatomical and functional peculiarities make wide excision difficult or impossible, requiring recourse to amputation in particular situations.
References
- Hao X, Billings SD, Wu F, Stultz TW, Procop GW, Mirkin G, et al. Dermatofibrosarcoma Protuberans: Update on the Diagnosis and Treatment. JCM. 2020; 9(6): 1752.
- Elafram R, Romdhane MB, Khessairi N, Sghaier M, Annabi H. Dermatofibrosarcoma protuberans of the hallux: A case report with review of the literature. International Journal of Surgery Case Reports. 2022; 96: 107325.
- Shah KK, McHugh JB, Folpe AL, Patel RM. Dermatofibrosarcoma Protuberans of Distal Extremities and Acral Sites: A Clinicopathologic Analysis of 27 Cases. American Journal of Surgical Pathology. mars 2018; 42(3): 413-9. 4.
- Darier J, Ferrand M. Dermatofibromes progressifs et récidivants ou fibrosarcomes de la peau. Ann Dermatol Syphiligr (Paris). 1924.
- Lemm D, Mügge LO, Mentzel T, Höffken K. Current treatment options in dermatofibrosarcoma protuberans. J Cancer Res Clin Oncol. 2009; 135(5): 653-65.
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma Protuberans. Dermatologic Clinics. 2019; 37(4): 483-8.
- Cabral R, Wilford M, Ramdass M. Dermatofibrosarcoma protuberans associated with trauma: A case report. Mol Clin Oncol. 2020; 13(5): 1-1.
- Bague S, Folpe AL. Dermatofibrosarcoma Protuberans Presenting as a Subcutaneous Mass: A Clinicopathological Study of 15 Cases With Exclusive or Near-Exclusive Subcutaneous Involvement. The American Journal of Dermatopathology. 2008; 30(4): 327-32.
- Acosta AE, Vélez CS. Dermatofibrosarcoma Protuberans. Curr Treat Options in Oncol. 2017; 18(9): 56.
- Bouhani M, Fertani Y, Zemni I, Adouni O, Bouida A, Chargui R, et al. Dermatofibrosarcoma Protuberans of the Breast in Man: An Extremely Rare Entity With a Review of the Literature. Journal of Investigative Medicine High Impact Case Reports. janv 2019; 7: 232470961987563.
- Bogucki B, Neuhaus I, Hurst EA. Dermatofibrosarcoma Protuberans: A Review of the Literature. Dermatologic Surgery. 2012; 38(4): 537-51.
- Navarrete-Dechent C, Mori S, Barker CA, Dickson MA, Nehal KS. Imatinib Treatment for Locally Advanced or Metastatic Dermatofibrosarcoma Protuberans: A Systematic Review. JAMA Dermatol. 2019; 155(3): 361.
- Takahira T, Oda Y, Tamiya S, Higaki K, Yamamoto H, Kobayashi C, et al. Detection of COL1A1-PDGFB fusion transcripts and PDGFB/PDGFRB mRNA expression in dermatofibrosarcoma protuberans. Mod Pathol. juin 2007;20(6):668-75.
- Sirvent N, Maire G, Pedeutour F. Genetics of dermatofibrosarcoma protuberans family of tumors: From ring chromosomes to tyrosine kinase inhibitor treatment. Genes Chromosom Cancer. 2003; 37(1): 1-19.
- Stamatakos M, Fyllos A, Siafogianni A, Ntzeros K, Rozis M, Kontzoglou K. Dermatofibrosarcoma protuberans: a rare entity and review of the literature.
- Boukovalas S, Castillo AC, Andry D, Lombana N, Murphy KD. Dermatofibrosarcoma Protuberans: Trauma and Genetics. 2017; 1(1).
- Chang JB, Kung TA, Cederna PS. Acute Marjolin’s Ulcers: A Nebulous Diagnosis. Annals of Plastic Surgery. 2014; 72(5): 515-20.
- Rasheed AA, Barwad A, Dhamija E, Garg R, Pandey R, Shamim SA, et al. Advanced dermatofibrosarcoma protuberans: an updated analysis of cases from an Indian sarcoma clinic. Future Science OA. 2021; 7(9): FSO743.
- Penel N, El Bedoui S, Robin YM, Decanter G. Dermatofibrosarcome : prise en charge. Bulletin du Cancer. nov 2018; 105(11): 1094-101.
- Thway K, Noujaim J, Jones RL, Fisher C. Dermatofibrosarcoma protuberans: pathology, genetics, and potential therapeutic strategies. Annals of Diagnostic Pathology. 2016; 25: 64-71.
- Chen X, Chen YH, Zhang Y li, Guo Y min, Bai Z lan, Zhao X. Magnetic resonance imaging and mammographic appearance of dermatofibrosarcoma protuberans in a male breast: a case report and literature review. J Med Case Rep. 2009; 3(1): 8246.
- Escobar GF, Ribeiro CK, Leite LL, Barone CR, Cartell A. Dermoscopy of Dermatofibrosarcoma Protuberans: What Do We Know? Dermatol Pract Concept. 2019; 139-45.
- Chen YT, Tu WT, Lee WR, Huang YC. The efficacy of adjuvant radiotherapy in dermatofibrosarcoma protuberans: a systemic review and meta-analysis. J Eur Acad Dermatol Venereol. 2016; 30(7): 1107-14.

