Review article - Volume 3 - Issue 2
Meta-analysis of clinical trials of transcranial magnetic stimulation for chronic pelvic pain syndrome
Mengyang W1,2; Rui X2; Shangjie C2; Min W1; Chunhua Y1; Yuxing K2; Xingjie L2; Ziyi W2; Hongdang Q1*
Director Environment Laboratories, Ras Al Khaimah Municipality, Ras Al Khaimah, United Arab Emirates.
Received Date : Feb 11, 2023
Accepted Date : Mar 06, 2023
Published Date: Mar 13, 2023
Copyright:© Hongdang Q 2023
*Corresponding Author : Hongdang Q, The First Affiliated Hospital of Bengbu Medical College, China.
Email: qhd820@sohu.com
DOI: Doi.org/10.55920/2771-019X/1393
Abstract
Introduction: In recent years, repeated transcranial magnetic stimulation has been used in clinical trials for the treatment of chronic pelvic pain syndrome, but its efficacy needs to be further verified. We aimed to summarize and discuss randomized controlled studies to provide clinical reference evidence for the future use of repeated transcranial magnetic stimulation in the treatment of chronic pelvic pain syndrome.
Material and methods: The protocol was registered in the PROSPERO database on 7 December 2021 (registration number: CRD42021284974). The PubMed, Cochrane Library, Embase, CNKI, VIP, Wanfang, and SinoMed databases were searched for all clinical trials published before 8 November 2021. The effect size was evaluated using the standardized mean difference and a 95% confidence interval.
Results: We included seven studies comprising 99 participants. Visual analog scales were used as the main indexes of pain intensity and no treatment effect was observed (standardized mean difference = 0.19, 95% CI -0.07 to 0.42, P = 0.13). A reduction in depression, anxiety, and symptoms was not found, but there was a significant effect on quality of life by SF-36 score (standardized mean difference = -8.69, 95% CI -12.04 to -5.35, P < 0.00001). No serious adverse events were reported within the included articles.
Conclusions: Transcranial magnetic stimulation may improve activities of daily living, and long-term interventions of repeated transcranial magnetic stimulation have a positive impact on pain and mood in patients with chronic pelvic pain syndrome. However, these findings should be interpreted with caution due to the limitations of the included studies.
Keywords: pelvic pain; TMS; meta-analysis; chronic pelvic pain syndrome; brain stimulation
Abbreviations
CPPS: Chronic pelvic pain syndrome
Hf-rTMS: High-frequency repetitive transcranial magnetic stimulation
MD: Mean difference
SMD: Standardized mean difference
TMS : Transcranial magnetic stimulation
rTMS: Repetitive transcranial magnetic stimulation
Introduction
Chronic pelvic pain is a group of diseases or complexes caused by multiple functional and/or organic causes and lasting for more than 6 months, with pain in the pelvis and surrounding tissues as the main symptom sign [1,2]. Chronic pelvic pain syndrome (CPPS) is a complex of conditions including endometriosis, irritable bowel syndrome, interstitial cystitis, and pudendal neuralgia that affects 15% to 25% of women [3,4], who are bothered not only by persistent severe pain but also by gastrointestinal problems and emotional changes. These conditions can seriously interfere with normal life and work. The complex long-term management of CPP keeps many women who are ill from going to the hospital, and research shows that CPPS imposes a heavy financial burden on women and international health systems [5].
Treatment of CPPS is usually based on the urinary, psychosocial, organ specific, infectious, neurological/systemic, and tenderness of skeletal muscles classification and requires multidisciplinary knowledge [6]. Patients with CPPS account for 12% of hysterectomies and 40% of laparoscopies each year, and only 10–15% of patients meet the surgical criteria. However, many patients may only recover after a long time and may have a series of complications after surgery, such as postoperative adhesion, organic pain, and infection, making rehabilitation more difficult [7-9]. Patients who continue to suffer from pain after medical and surgical treatment often choose to try manual, aerobic exercise, and cognitive therapy [10,11].
Physical therapy has produced significant results as a more comfortable treatment option. Among them, transcranial magnetic stimulation (TMS), as a noninvasive brain stimulation technique, is widely used in the treatment of complex neuropathic pain. The pathomechanism of CPPS may be due to abnormal central nervous sensitization and an imbalance of the excitatory and inhibitory systems [12]. Repetitive TMS (rTMS) may be considered a safe method to regulate cortical excitability and pain thresholds, and the analgesic effect of rTMS has been confirmed in some patients who are resistant to medical treatment, mentioned in a meta-analysis on rTMS for chronic neurological pain [13,14].
In a case report using rTMS to stimulate the primary motor cortex (M1) for refractory pelvic and perineal pain, Louppe et al. proposed the idea of treatment with neural regulation technique for the first time [15]. Some CPPS patients were still affected by pain after drugs, surgical treatment, and various peripheral treatments; however, 2 weeks after rTMS treatments, the pain was significantly relieved. Since then, continuous studies have proven the influence of rTMS on neuropathic pain in complex regions [16-19]. Recent systematic reviews have shown that rTMS has a positive effect on the treatment of chronic pain, mainly for psychological, gastrointestinal digestive, and urogenital system disorders [14].
The use of rTMS in the treatment of chronic pain is well studied and the efficacy and safety of treatment options are well established. However, there is currently no review of the efficacy of rTMS in treating CPPS. According to previous literature [20-23], there are differences in treatment outcomes, a variety of treatment regimens, and the choice of outcome assessment indicators is not constant. Therefore, we aimed to summarize and discuss these studies to provide clinical reference evidence for the future use of rTMS in the treatment of CPPS.
Material and Methods
Search strategy
The databases of PubMed, the Cochrane Library, Embase, CNKI, VIP, Wanfang, and SinoMed were searched for all clinical trials published before 8 November 2021. The search terms were “Transcranial magnetic stimulation, chronic pelvic pain syndrome,” and the search was limited to human studies. Manual searches of the reference lists of the pertinent articles were also conducted to identify relevant articles.
Study selection
Initial screening was based on titles and abstracts. Because CPPS covers a wide range of types, all of the articles that were included in the initial screening were retained if they met the disease types in the EAU guidelines. Two reviewers independently assessed these articles for eligibility. In cases of disagreement, the two reviewers checked the full text of the article and discussed it with each other to reach an agreement.
The articles were assessed, and studies were included if they met the following criteria: (1) belonged to a clinical trial, regardless of the type of trial; (2) did not include chronic myofascial syndrome not specified as a trigger point; (3) patients were adults (≥18 years); (4) results included at least pain scores; and (5) the type of intervention was rTMS.
Quality appraisal assessment
Each study was individually assessed by two reviewers (WMY and ZYW), who independently evaluated the risk of bias of included studies using the Cochrane Collaboration tool. The six recommended domains, involving seven items, included selective bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessors), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias. The overall judgment for each study was classified as “low,” “high,” or “unclear” based on the degree of bias. Discrepancies between reviewers were resolved by the third reviewer (RX).
Data extraction
The literature search, study identification, and data extraction processes were conducted between November 2021 and December 2021. All searched records were imported into the reference management software (EndNote x9) to eliminate duplicate records. The full texts of the studies that potentially met the inclusion criteria were obtained to further evaluate their eligibility. Any disagreements were resolved by discussion with the third reviewer (RX). Data were extracted by one reviewer (MYW) using the prepared form and checked for accuracy by another reviewer (ZXX).
The standard form was jointly designed by two reviewers to collect relevant data from each study to obtain the following information: (1) patient characteristics; (2) trial design; (3) rTMS treatment protocol; (4) outcome measures; and (5) the duration of follow up.
Statistical analyses
Review Manager software V.5.3 provided by Cochrane Collaboration was used for the statistical analysis, and the statistical significance was defined as a two-sided P-value of < 0.05. Data were summarized using relative risk with 95% CI for binary outcomes, as well as mean difference (MD) or standardized MD (SMD) and corresponding 95% CI for continuous outcomes. However, when the heterogeneity among studies was high (I2 > 75%), the overall pooled analysis was considered inappropriate, and the statistical heterogeneity among the included studies was considered very serious according to the causes of heterogeneity, including differences in assessment tools, subjects, and design of trial protocols. If the results were presented only graphically, the Get Data Graphic Digitizer 2.26 was used (https://getdata.com/) to extract the required data.
Ethics statement
Our meta-analysis followed the PRISMA statement and confirmed that all methods were followed under PRISMA guidelines and regulations (Supplementary material: PRISMA-2020-checklist). The protocol of this study was registered at the International Prospective Register of Systematic Review, PROSPERO, under the identification CRD42021284974 and can be integrally assessed online (https://www.crd.york.ac.uk/prospero/#recordDetails).
Results
Study identification
Of the 217 studies found after the initial database search, seven were identified for further analysis (N = 99). The flow chart of the selection process is shown in Figure 1. Four studies came from France, the rest were from the United Kingdom, Finland, and Italy. The number of participants ranged from 30 to 76 years, and the duration of illness ranged from 1.4 to 28.5 years. In total, 66.6% of study participants were female.
TMS was applied in all included studies [15,20-25]. Three studies were of patients with irritable bowel syndrome [20,23,24];
Table 1: Characteristics of the selected studies.
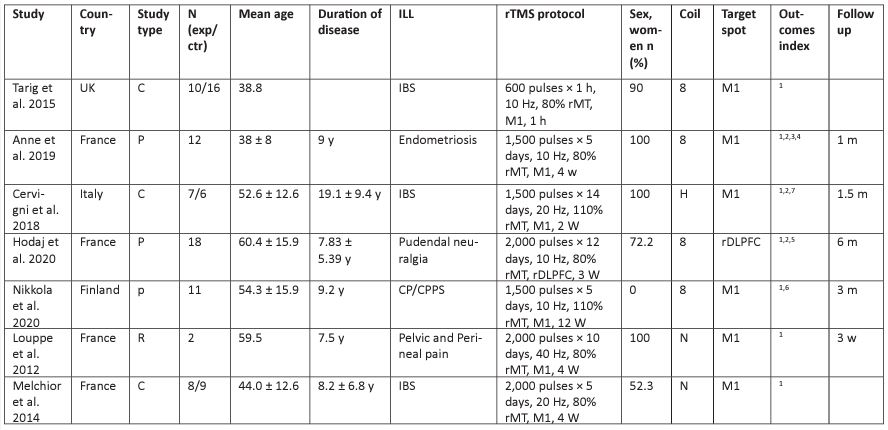
two, with pudendal neuralgia [15,21]; one, with endometriosis [22]; and one study was of patients with CP/CPPS [25]. Except for one case report [15], all other studies were part of parallel sham control and crossover sham control trials. Only one study used rTMS as a single treatment; the crossover sham control trial results only took data before uncrossing [20]. In one of the studies [24], patients received 1 hour of stimulation, and the remaining studies were treated for an average of 4.83 weeks. All studies were published in English. The details of the included studies and the results of quality assessments are shown in Table 1.
Among the studies, six chose M1 as the brain region of TMS, and seven studies used high-frequency stimulation. There were two articles with a stimulation intensity of 110% rMT and five articles with 80% rMT. The figure-of-eight coil was used in four studies; one used an H-coil [20]; and the remaining two did not reported which coil was used. Five studies included a follow up.
Risk of bias assessment
Two articles reported a method for random sequence generation (Figure 2) [20,23]. Five did not state whether randomization was concealed. The participants or research team were not blind in two surveys and the blind method was not mentioned in detail in two reports. In five studies, there was no explanation for blinding in the outcome assessment. Two studies had incomplete reporting of results, and two studies had missing follow up and incomplete outcome data, with many deviations [15,24].
Pain intensity
Seven comparisons from the seven surveys involved pain intensity, all of which were meta-analyzed by all comparisons of the visual analog scale pain scale, and the results showed that there was no significant change after treatment (n = 176, SMD = 0.18, 95% CI 0.07 to 0.42, P = 0.15; I2 = 75%; the fixed-effect model; Figure 3a). In one of the studies, the intervention was 1 hour long; we performed the analysis again after excluding this study, and the results were as follows: n = 156, SMD = 1.48, 95% CI 0.8 to 2.16, P < 0.0001, I2 = 62%; Figure 3b). Two out of seven studies had pain outcomes at 1 month, which was not significant (SMD = 0.69, 95% CI -0.22 to 1.60, I2 = 0.0%; Figure 3c).
Symptoms
Three studies reported the effects of rTMS on symptoms measured with the EPH-30 [26], NIH-CPSI [27], and OAB-q [28] in subjects with CPPS, endometriosis, CP/CPPS, and IBS. Therefore, due to the use of outcome measurement tools not used in the study, the results are incompatible, and the overall pooled analysis was not appropriate (Figure 4).
Emotional effect
Two studies reported complete mood treatment results, using the Beck Scale [29]. and the effects of rTMS measured by the HAMD [30]. on anxiety vs depression in subjects, respectively (Figure 5).
Quality of life
There were four articles mentioning the SF-36. Three of these studies recorded SF-36 [31] scores after treatment, and the control groups showed a large decrease (SMD, 8.69, 95% CI 12.04 to 5.35, I2 = 3%; Figure 6).
Subgroup analysis
Five studies used interventions of 80% rMT intensity, four of which were longer than 2 weeks, and pain score was used as the evaluation index (SMD = 1.39, 95% CI 0.64 to 2.14, I2 = 54%; Figure 7d). The intensity of the two stimuli was 110% rMT, and the results of the meta-analysis were as follows: SMD = 1.89, 95% CI 0.26 to 3.51, I2 = 84%; Figure 7e). The confidence interval and heterogeneity of the high-intensity group were higher than those of the low-intensity group, which may be related to the difference in outcome calculation standards and case inclusion baseline. The meta-data of the two groups cannot directly prove that the high-intensity group was superior to the low-intensity group.
Discussion
We systematically reviewed seven clinical trials investigating the effects of rTMS on CPPS. Patients receiving repetitive rTMS had higher SF-36 scores and lower pain compared to controls. These effects lose significance 1 month after the last treatment. In addition, there was no reduction in anxiety and depression, no reduction in various symptom scores, and a new non-significant correlation between pain severity and rTMS stimulation parameters. We found that pain intensity, as measured by visual analog scale scores, improved in repeated stimulation studies and that improvement was also significant in a single case report [32] that did not meet the criteria of the meta-analysis.
Considering that most of the articles had an inferior double-blind effect, the trial allocation was not randomized, and the resulting outcome measures were quite different. The heterogeneity of the results of this study is generally not applicable because the anxiety, depression, and symptom evaluations cannot be globally evaluated. Moreover, different measurement tools are used in each survey.
In our analysis, six studies chose the motor cortex [15, 20, 22-25] and only one was conducted on the prefrontal cortex [21]. All seven studies performed their analysis using Hf-rTMS as single or repetitive stimulation. Several studies also confirmed the more significant effect of Hf-rTMS stimulation of the M1 region on chronic pain [31-36]. Additionally, there was a systematic review [37] that studied the effect of Hf-rTMS stimulation of the dorsolateral prefrontal cortex on chronic pain, and showed the short-, mid-, and long-term analgesic effects of TMS on neuropathic pain in the DLPFC. Therefore, the results of the follow-up period were presented; however, due to the incomplete recording of data, there was no significant difference in pain intensity 1 month after the last treatment.
A recent meta-analysis concluded that Hf-rTMS has therapeutic implications for the motor cortex for a variety of diseases, including not only chronic pain but also depression and anxiety. In several studies [38,39], symptom scores were not limited to the evaluation of the disease itself, but also included gastrointestinal and urinary function evaluations. Therefore, the analysis of the results of this study used the pain score as the primary measure and included mood, symptoms, and quality of life evaluations. However, the exact mechanism of action by which rTMS affects pain is unknown. The mechanism of labor pain in the M1 and DLPFC [40] area by TMS may involve direct inhibition of spinal transmission of nociceptive signals. A review, showing rTMS of prominent axons and local interneurons activated at high frequencies (10 or 20 Hz), established that cumulative pain can be reduced for at least a few weeks after 10 consecutive working days. The pain-reducing effect of TMS is also considered to be mediated through subcortical neural networks and is the result of enhancement of the dopamine-opioid system, and it has also been reported that TMS therapy can increase serum-endorphin concentrations [16, 19, 34].
Through the analysis of stimulation parameters, all studies involved high-frequency stimulation, of which the intensity was 110% rMT in two studies and 80% rMT in five studies. The study by Zheng et al [41]. showed that rTMS had a more significant effect on cerebral blood flow at high intensity, and the intensity effect was greater than the frequency effect. However, in the results of this meta-analysis, the 95% confidence interval of the high-intensity group was larger than that of the low-intensity group; the difference was not significant, and the pain intensity of the experimental group was reduced after repeated stimulation. However, it cannot be stated that the strength does not have an effect on the difference in the results, mainly because the included article is not a randomized controlled trial with a strictly designed protocol.
The results of this article include two studies of the relationship between pain and bowel sensation during TMS. One study measured rectal and anal pain and sensory thresholds and concluded that rTMS at 10 Hz appears to change anal and rectal pain with little effect on anorectal sensation, and the other study concluded that TMS of the primary motor cortex improves maximum rectal tolerance in IBS patients with significant allergies. Both of these studies provide a basis for IBS in neurostimulation therapy and also lay the foundation for the improvement of CPPS in terms of gastrointestinal conditions.
Two studies reported mild and transient headaches, nausea, inappropriate site of stimulation, and some neurobehavioral adverse events, which were common adverse reactions to TMS and resolved spontaneously in a short period. There were no serious adverse events reported, and the serious adverse event of TMS, epilepsy, was not observed.
The strengths of our study lie in several aspects. First, we present the first extensive summary of rTMS for the treatment of CPPS. Second, we show the comparative analysis of rTMS in the treatment of CPPS under different intensity stimulation parameters for the first time. Third, we included the latest clinical trial articles, providing a reference basis for subsequent treatment and research.
The present meta-analysis has several limitations. First, the number of participants in all included studies was small, and patient demographics, study design, and stimulation parameters were heterogeneous. Second, all included studies had a female preponderance. Although no correlation between treatment effect and gender was found in the meta-analysis, the incidence of CPPS in men was not low, but that study was biased toward medical and surgical treatment. Third, the diagnostic inclusion and evaluation criteria used were different. CPPS includes diverse diseases, and three similar studies had different symptom diagnoses and classifications. Fourth, most of the included surveys allowed concurrent medication and other treatments during the study period. Therefore, most TMS is used as an adjuvant therapy, and the results record interactions between drugs and other treatments, and the effectiveness of rTMS alone needs further validation. Fifth, due to insufficient data and high heterogeneity among studies, we failed to complete the group analysis of stimulus flapping, adjuvant therapy for participants, its relationship with symptom improvement, and adverse effects. However, these results are important to assess the treatment of chronic pain [42], and a large number of trials are needed in the future to confirm the results in this regard. Finally, although no serious adverse events were reported, the relatively small number of participants in most studies may have affected the incident rate and needs to be illustrated by studies based on large samples for a long time with a single disease.
Future research in this field should still be the focus, and many trials with perfect standards are needed. For example, the CPPS diagnostic criteria and treatment recommendations of the EAU guidelines [7] are uniformly used; studies should be based on a reasonable and statistically significant sample size and a more objective randomized design trial is conducted; it is recommended to use the CPPS pain and urinary symptom scoring scale, and male and female CPPS measurement tools recommended by the specifications according to gender differences; and there is also a great need for mechanistic studies of cortical and peripheral mechanisms.
Conclusion
This meta-analysis revealed that rTMS is safe and effective to treat multiple domains of CPPS but has no significant effects on symptoms or emotional aspects. This may be due to improper experimental design and minimal research data. Therefore, large-scale trials using rigorously designed and standardized training protocols are still needed to investigate the future of rTMS for CPPS.
Conflicts of Interest statement
The authors declare no competing interests.
Funding information|
National Natural Science Foundation of China (81973922)
Declarations of interest
This review received no financial or non-financial support.
Data availability statements
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgements
We wish to thank the teachers and colleagues who helped to write the manuscript.
References
- Speer LM, Mushkbar S, Erbele T. Chronic pelvic pain in women. Am Fam Physician. 2016; 93: 380-687.
- Dydyk AM, Gupta N. Chronic pelvic pain. In: StatPearls [Internet]. StatPearls Publishing. 2021.
- Till SR, As-Sanie S, Schrepf A. Psychology of chronic pelvic pain: prevalence, neurobiological vulnerabilities, and treatment. Clin Obstet Gynecol. 2019; 62: 22-36.
- Mardon AK, Leake HB, Chalmers KJ. A review of chronic pelvic pain in women. JAMA. 2021; 326: 2206.
- Huang G, Le AL, Goddard Y, et al. A Systematic review of the cost of chronic pelvic pain in women. J Obstet Gynaecol Can. 2022; 44: 286-293.
- Magri V, Wagenlehner F, Perletti G, et al. Use of the UPOINT chronic prostatitis/chronic pelvic pain syndrome classification in European patient cohorts: sexual function domain improves correlations. J Urol. 2010; 184: 2339-2345.
- Fall M, Baranowski AP, Elneil S, et al. EAU guidelines on chronic pelvic pain. Eur Urol. 2010; 57: 35-48.
- Wozniak S. Chronic pelvic pain. Ann Agric Environ Med. 2016; 23: 223-226.
- Smith SE, Eckert JM. Interventional pain management and female pelvic pain: considerations for diagnosis and treatment. Semin Reprod Med. 2018; 36: 159-163.
- Nygaard AS, Rydningen MB, Stedenfeldt M, et al. Group-based multimodal physical therapy in women with chronic pelvic pain: a randomized controlled trial. Acta Obstet Gynecol Scand. 2020; 99: 1320-1329.
- Franco JV, Turk T, Jung JH, et al. Non-pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome. Cochrane Database Syst Rev. 2018; 5: Cd012551.
- Doiron RC, Nickel JC. Management of chronic prostatitis/chronic pelvic pain syndrome. Can Urol Assoc J. 2018 ;12: S161-S163.
- Jin Y, Xing G, Li G, et al. High frequency repetitive transcranial magnetic stimulation therapy for chronic neuropathic pain: a meta-analysis. Pain Physician. 2015; 18: E1029-1046.
- O’Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2018 ;4: Cd008208.
- Louppe JM, Nguyen JP, Robert R, et al. Motor cortex stimulation in refractory pelvic and perineal pain: report of two successful cases. Neurourol Urodyn. 2013; 32: 53-57.
- Lefaucheur JP. Cortical neurostimulation for neuropathic pain: state of the art and perspectives. Pain. 2016; 157: S81-S89.
- Moisset X, Lanteri-Minet M, Fontaine D. Neurostimulation methods in the treatment of chronic pain. J Neural Transm (Vienna). 2020; 127: 673-686.
- Moisset X, Lefaucheur JP. Non pharmacological treatment for neuropathic pain: invasive and non-invasive cortical stimulation. Rev Neurol (Paris). 2019; 175: 51-58.
- Lefaucheur JP, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018). Clin Neurophysiol. 2020; 131: 474-528.
- Cervigni M, Onesti E, Ceccanti M, et al. Repetitive transcranial magnetic stimulation for chronic neuropathic pain in patients with bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. 2018; 37: 2678-2687.
- Hodaj H, Payen JF, Hodaj E, et al. Long-term treatment of chronic orofacial, pudendal, and central neuropathic limb pain with repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol. 2020; 131: 1423-1432.
- Pinot-Monange A, Moisset X, Chauvet P, et al. Repetitive transcranial magnetic stimulation therapy (rTMS) for endometriosis patients with refractory pelvic chronic pain: a pilot study. J Clin Med. 2019; 8: 508.
- Melchior C, Gourcerol G, Chastan N, et al. Effect of transcranial magnetic stimulation on rectal sensitivity in irritable bowel syndrome: a randomized, placebo-controlled pilot study. Colorectal Dis. 2014; 16: O104-O111.
- Algladi T, Harris M, Whorwell PJ, Paine P, Hamdy S. Modulation of human visceral sensitivity by noninvasive magnetoelectrical neural stimulation in health and irritable bowel syndrome. Pain. 2015; 156: 1348-1356.
- Nikkola J, Holm A, Seppänen M, Joutsi T, Rauhala E, Kaipia A. Repetitive transcranial magnetic stimulation for chronic prostatitis/chronic pelvic pain syndrome: a prospective pilot study. Int Neurourol J. 2020; 24: 144-149.
- Chauvet P, Auclair C, Mourgues C, Canis M, Gerbaud L, Bourdel N. Psychometric properties of the French version of the Endometriosis Health Profile-30, a health-related quality of life instrument. J Gynecol Obstet Hum Reprod. 2017; 46: 235-242.
- Leskinen MJ, Mehik A, Sarpola A, Tammela TL, Järvelin MR. The Finnish version of The National Institutes Of Health Chronic Prostatitis Symptom Index correlates well with the visual pain scale: translation and results of a modified linguistic validation study. BJU Int. 2003; 92: 251-256.
- Coyne KS, Thompson CL, Lai JS, Sexton CC. An overactive bladder symptom and health-related quality of life short-form: validation of the OAB-q SF. Neurourol Urodyn. 2015; 34: 255-263.
- Steer RA, Clark DA, Beck AT, Ranieri WF. Common and specific dimensions of self-reported anxiety and depression: the BDI-II versus the BDI-IA. Behav Res Ther. 1999; 37: 183-190.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983; 67: 361-370.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992; 30: 473-483.
- Nizard J, Esnault J, Bouche B, Suarez Moreno A, Lefaucheur JP, Nguyen JP. Long-term relief of painful bladder syndrome by high-intensity, low-frequency repetitive transcranial magnetic stimulation of the right and left dorsolateral prefrontal cortices. Front Neurosci. 2018; 12: 925.
- Nguyen JP, Dixneuf V, Esnaut J, et al. The value of high-frequency repetitive transcranial magnetic stimulation of the motor cortex to treat central pain sensitization associated with knee osteoarthritis. Front Neurosci. 2019; 13: 388.
- Klein MM, Treister R, Raij T, et al. Transcranial magnetic stimulation of the brain: guidelines for pain treatment research. Pain. 2015; 156: 1601-1614.
- Granovsky Y, Sprecher E, Sinai A. Motor corticospinal excitability: a novel facet of pain modulation? Pain Rep. 2019; 4: e725.
- Zhang KL, Yuan H, Wu FF, et al. Analgesic effect of noninvasive brain stimulation for neuropathic pain patients: a systematic review. Pain Ther. 2021; 10: 315-332.
- Che X, Cash RFH, Luo X, et al. High-frequency rTMS over the dorsolateral prefrontal cortex on chronic and provoked pain: a systematic review and meta-analysis. Brain Stimul. 2021; 14: 1135-1146.
- Nardone R, Versace V, Sebastianelli L, et al. Transcranial magnetic stimulation and bladder function: a systematic review. Clin Neurophysiol. 2019; 130: 2032-2037.
- Vacher P, Charlanes A, Chesnel C, et al. [Interest of transcranial stimulation in pelvic and perineal disorders]. Prog Urol. 2019; 29: 349-359.
- Dall’Agnol L, Medeiros LF, Torres IL, et al. Repetitive transcranial magnetic stimulation increases the corticospinal inhibition and the brain-derived neurotrophic factor in chronic myofascial pain syndrome: an explanatory double-blinded, randomized, sham-controlled trial. J Pain. 2014; 15: 845-855.
- ZHENG XK, HE X, YAN TB, DONG JT. A realtime observation on cerebral blood flow of normal rats affected by transcranial magnetic stimulation. Chin J Rehab Med. 2015; 30: 417-21.
- Patel KV, Amtmann D, Jensen MP, Smith SM, Veasley C, Turk DC. Clinical outcome assessment in clinical trials of chronic pain treatments. Pain Rep. 2021; 6: e784

