Review Article - Volume 3 - Issue 2
The Role of T Cells and Innate Immunity in Long-Term Immunity and Vaccine Development for COVID-19
Esmaeil F*
Peace and Health Organization, San Diego, California, USA.
Medical Research Center, the University of Tennessee, Knoxville, USA.
Received Date : Feb 17, 2023
Accepted Date : April 17, 2023
Published Date: Mar 24, 2023
Copyright:© Esmaeil F 2023
*Corresponding Author : Esmaeil F, Peace and Health Organization, San Diego, California, USA, Medical Research Center, the University of Tennessee, Knoxville, USA.
Email: farshi@technologist.com
DOI: Doi.org/10.55920/2771-019X/1402
Abstract
Coronaviruses are significant pathogens for humans. Our findings suggest that antibodies may not have a crucial role in long-term immunity against COVID-19, but T cells, a type of white blood cell, could play a critical role. T cells have long-term memory in the blood. Investigating mild cases of COVID19 in children is key to understanding the disease, as it may uncover important protective mechanisms and therapeutic targets. Children’s milder response to COVID-19 compared to adults suggests that children have a higher resistance to the virus due to their innate immune system. Our results indicate that phagocytes, a component of the innate immune system, play a significant role in eliminating COVID-19 in both mice and humans. We also found that CD4+ T cells activate B cells and play a crucial role in primary infections. Kids have higher levels of natural antibodies (IgM and IgG) compared to adult patients, and the number of γδ T cells increases both locally and systemically in kids with COVID-19, but decreases in adults with severe symptoms. Our observations have important implications for developing novel vaccines and therapies for COVID-19. To produce a vaccine, the following factors must be considered: 1) phagocytes, 2) natural antibodies, 3) T cells, and 4) white blood cells. The vaccine should be based on T cells instead of antibodies and should also boost the innate immune system, including phagocytes. A novel vaccine eliciting a protective immune response against SARS-Cov-2 is suggested.
Introduction
Coronaviruses are significant pathogens for humans and animals. In late 2019, a novel coronavirus was identified as the cause of pneumonia outbreaks in Wuhan, China and rapidly spread resulting in a global epidemic [31]. This new virus, called COVID-19, led to millions of cases and deaths, with symptoms including high fever, cough, and shortness of breath. The COVID-19 virus is a beta coronavirus similar to the SARS-CoV virus and may use the same receptor for cell entry [1]. It is also 96% identical to a bat coronavirus at the wholegenome level [2-8]. Effective treatments and vaccines for COVID-19 are still needed and require understanding of how host immune responses control the infection. Evidence suggests recovered COVID-19 patients have acquired immunity based on T and B cells, but the exact mechanism remains unclear. The immune system is divided into two types: innate and adaptive. The innate system quickly detects and responds to common infections but is limited in specificity. It signals the adaptive system, which provides a more target-specific defense through B and T cells [8]. T cells are further divided into CD4+ and CD8+ cells, with CD4+ releasing cytokines to activate B cells and produce antibodies, while CD8+ directly kill infected cells. The adaptive system also creates memory T and B cells for longterm protection. People infected with COVID-19 are able to produce specific T and B cells, but the exact adaptive immune response to the novel coronavirus is not yet well understood.
Disappearing Antibodies
The COVID-19 pandemic has shown conflicting results regarding the susceptibility of children to the virus compared to adults. Children's immune systems are dominated by innate immunity, whereas adult immune systems rely more on antibodies. This difference may help with developing vaccines and therapies. Our research has shown that T cells play a role in controlling COVID-19infection, as well as innate defense mechanisms demonstrated by clearance of the virus in mice depleted of CD4+ and CD8+ T cells [13]. A study also reported the importance of CD4+T cells in controlling COVID-19 [14]. However, a lack of activation of innate immunity and a barely detectable antivirus T cell response was seen in mice infected with a mouse-adapted strain of COVID-19 [9]. On the other hand, aged mice infected with a human clinical isolate of COVID-19successfully eliminated the virus, serving as a model for host immune responses in clearing COVID-19.
According to the pathological reports for COVID-19, it was shown that COVID-19 mainly caused inflammatory responses in the lungs [7]. Several studies showed that COVID-19 patients developed lymphopenia and rising pro- inflammatory cytokines in severe cases [8, 9].
Inflammation can be triggered when innate and adaptive immune cells detect COVID-19 infection. Innate T cells can provide a first line of defense against pathogens. However, how innate T cells respond to COVID-19 infection remains unclear.
Mice model
The contribution of cellular versus humoral immune responses in the resolution of acute COVID-19 infection has not yet been fully understood. In our study, we aimed to determine immune responses during acute COVID-19 infection by analyzing viral titers in the lungs of aged BALB/c mice (>8 months old), young BALB/c mice (<4 weeks old), and SCID mice (8 weeks old) after infection with acute COVID-19 from a 42-year-old male patient's throat lavage. The mice were divided into four groups each: control, CD8+ T cell depleted, CD4+ T cell depleted, and CD20+ B cell depleted, with the latter two depleted 7 days before infection. Results showed that aged BALB/c mice had higher titers compared to young BALB/c mice and SCID mice, which did not show signs of pneumonia. SCID mice, lacking T and B cells, were persistently infected, but adoptive transfer of splenocytes from BALB/c mice accelerated the elimination of COVID-19 in SCID mice. Depletion of CD4+ or CD8+ cells in BALB/c mice led to higher viral loads and demonstrated the importance of CD4+ T cells for controlling COVID-19. B cell activation by CD4+ T cells was also essential for elimination. Our findings have important implications for COVID-19 vaccination strategies and demonstrate the role of phagocytosis as a first line defense mechanism.
Other blood cells also may serve as effectors for the control of COVID-19 Specifically, elevated levels of alveolar macrophages, monocyte-derived infiltrating macrophages, and neutrophils that were observed also in many SARS patients [18]. To investigate the role of these myeloid cells in the clearance of COVID-19-infected pulmonary tissue, each subset of these myeloid cells was depleted by administration of a specific mAb or reagent. Consistent with other reports in SARS [19], alveolar macrophages were depleted for more than 5 days following in administration of 100 μL of 33% clodronate liposome. The observations of this study highlight the importance of cellular and humoral immune responses in the resolution of acute COVID-19 infection. The results demonstrate that CD4+ T cells play a crucial role in controlling COVID-19 infection, both directly and indirectly. The elimination of the virus requires the activation of B cells by CD4+ T cells, and CD8+ T cells are also an essential cell type for the control of COVID-19. Additionally, phagocytosis was found to be an important first line of defense against invading pathogens, and plays a role in the continuous clearance of dying cells and tissue remodeling. These findings have important implications for the development of novel vaccination strategies to alleviate COVID-19 associated diseases. The study highlights the importance of continued research into the immune responses involved in the resolution of acute COVID-19 infection, as this knowledge will be crucial in the development of effective treatments and preventative measures for this virus.
Human model
Phagocytosis plays a crucial role in the immune system, performed by various cells such as neutrophils, macrophages, dendritic cells, and B lymphocytes. The majority of COVID-19 cases are asymptomatic or mild to moderate, but approximately 15% progress to severe pneumonia and 5% develop ARDS, septic shock, or multiple organ failure. Despite this, there is limited research on the role of phagocytic cells in COVID-19. In our study, we investigated 13 children aged 4-10 with early COVID-19 infection and 33 adults aged 18-67 with severe COVID-19 infection. We analyzed both innate and adaptive immune responses in all patients [24]. The serocon version curves revealed that total antibodies and IgM and IgG were 100% detectable a month after symptom onset, but less than 40% of patients tested had antibodies in the first week [28]. We compared IgM and IgG levels between children and adults. Our results showed that children had higher levels of natural antibodies, mostly IgM, during the early stages of infection [28]. This is because natural antibodies, which are generated independently of previous antigen encounters, play a crucial role in controlling the infection before high-affinity antibodies and MBCs can be produced. In humans, natural antibodies are produced by innate or IgM MBCs, which are most abundant in children. Our results showed that adult patients with severe COVID-19 had lower CD4+ and CD8+ lymphocyte populations, which are strong predictors of in-hospital mortality, organ injury, and severe pneumonia. These patients also had lower total T cell counts, both helper T cells and suppressor T cells, and increased cytokine levels (IL-6, IL10, TNFα) and lymphopenia. The surviving T cells in severe COVID-19 cases appear to be dysfunctional [28, 32]. Cytotoxic T-cells and Natural Killer cells are important for generating an effective immune response against viruses in humans [33]. Our study found that increased cytokine levels and lymphopenia (significantly reduced CD4+ and CD8+ T cells) correlated with disease severity of COVID-19. Patients with severe COVID-19 also had lower lymphocyte and higher neutrophil counts in their blood, specifically lower CD8+ lymphocytes and NK cells compared to those with mild infection or healthy individuals. Among innate immune cells, γδ T cells are known for their
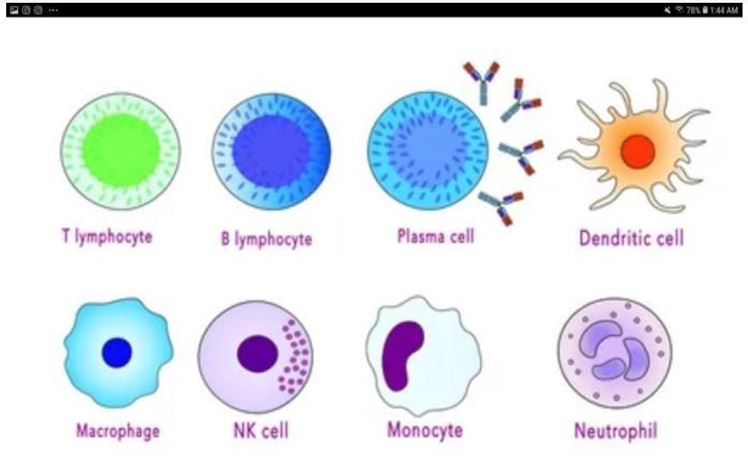
Figure 1: T lymphocytes, B lymphocytes, Plasma cell, Dendritic cell, Marcophage, NK cell, Monocyte, Neutrophil.
Figure 2: SARS-Cov-2 spike and T cell.
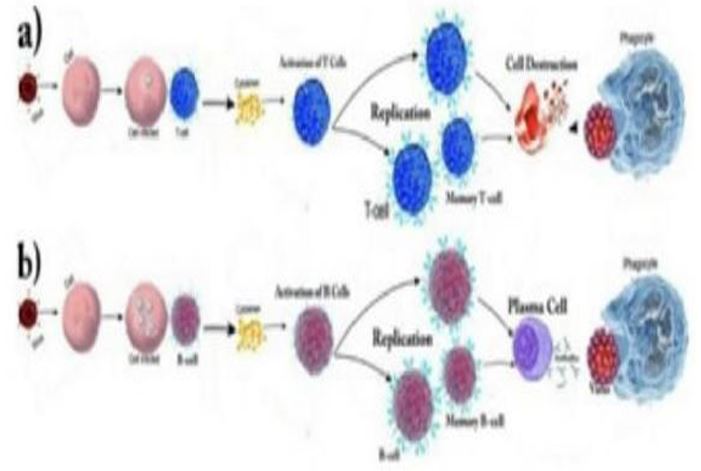
Figure 3: Scenario for infection of cell by SARS-Cov2 and production and activation of a)T cell b)B cell
rapid proliferation and ability to induce apoptosis, present antigens, and regulate the immune system in response to pathogens [10]. In healthy adults, γδ T cells make up 1-10% of circulating lymphocytes, primarily expressing the CD4 and CD8 double negative phenotype, although some can express CD4 or CD8 [11,12]. Unlike other T cells, γδ T cells do not recognize peptide antigens and their T cell receptors are not restricted by MHC, allowing them to respond to pathogen-associated molecular patterns and produce cytokines without TCR ligands
Figure 4: Antibodies of SARS-Cov-2 from ref [34].
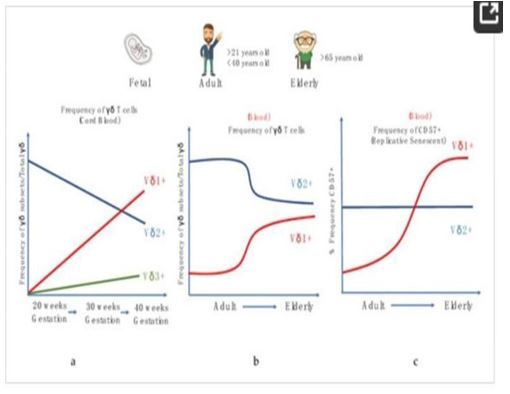
Figure 5: Alterations in human γδ T cells during development and aging. (a) Frequency of γδ sub-sets/Total γδ in the cord blood during gestational weeks, (b) Frequency of γδ subsets/ total γδ in the periphery with age. (c) Frequency of CD57+ γδ cell populations in the periphery with age.
[15]. γδ T cells are known to increase in number postinfection, playing an important role in the host immune response [12-14]. In many infections, the number of γδ T cells increases both locally and systemically a few days’ post-infection. A study found that the ratio of γδ T cells among total lymphocytes in the lungs significantly increased in mice infected with influenza A (H1N1) virus three days’ post infection [16,17]. To examine the behavior of γδ T cells during COVID-19 infection, PBMC samples from 38 patients were analyzed. The results showed that the percentage of γδ T cells in the peripheral blood of COVID-19 patients was drastically reduced compared to healthy donors. Unlike in other viral infections where γδ T cells increase in percentage, the decrease of γδ T cells in symptomatic COVID-19 patients might be due to the different ways various viruses impact γδ T cells [19]. The data also showed that the percentage of CD4 γδ T cells increased dramatically in COVID-19 patients, indicating a potential role in antigen presentation and activation of adaptive immune cells. This suggests that γδ T cells may act as a bridge between innate and adaptive immunity in response to COVID-19 [20]. In COVID-19 patients, γδ T cells were observed to exhibit a strong activation phenotype based on CD25 expression. However, the early activation marker CD69 showed no difference between patients and healthy donors, potentially due to early expression followed by reversion to the quiescent state during recovery. Although a decreased percentage of γδ T cells was observed, the expression of PD-1 did not differ between patients and healthy donors, suggesting γδ T cells did not undergo exhaustion. "In summary, γδ T cells play a crucial role in the immune response against COVID-19 infection by immediately responding to the virus and upregulating the activation marker CD25. They work in parallel with other innate cells to provide both direct and indirect defenses against the virus. Furthermore, the increased expression of CD4 in γδ T cells may serve as a biomarker for the assessment of COVID-19 infection. In a study, it was discovered that γδ T cells can also act as professional phagocytes, a function previously thought to be exclusive to myeloid cells. The study used confocal microscopy, transmission electron microscopy, and functional Ag presentation assays to show that freshly isolated human peripheral blood γδ T cells can phagocytose and process Escherichia coli. These findings support the idea that γδ T cells are evolutionarily ancient lymphocytes and provide insights into their role in transitional immunity and control of infectious diseases and cancer. They may act as a bridge between innate and adaptive immunity in response to COVID-19 infection. Additionally, T cells from recovered patients have been shown to target the virus, which is promising for vaccine development and indicates normal, good antiviral immunity."
Disappearing Antibiotics
Our studies have shown that COVID-19 antibodies can be detected in recovered patients for about 8 weeks, but the precise timeline is unclear due to the variability of symptoms and immune responses among patients. A comparison between symptomatic and asymptomatic people revealed that asymptomatic individuals had lower antibody levels, with about 40% having no detectable antibodies after 8 weeks. These findings suggest that antibodies to COVID-19 may not persist for long, however, the presence of memory T and B cells capable of reactivating to protect against reinfection cannot be excluded. The antibodies made by the immune system's B cells may disappear in a few weeks, but the memory cells generated persist for much longer. Our results also show that T cells from recovered patients can target the virus, compensating for the disappearance of antibodies and offering promising news for vaccine developers as this is consistent with normal, good, antiviral immunity
Discussion
The COVID-19 pandemic has resulted in over several hundred million cases since December 2019, with a mortality rate of 3%. The worst symptoms of the disease appear to be associated with agedependent immune response defects, as the mortality rate was over 50% for patients over 65 years of age. Retrospective studies of recovered COVID-19 patients suggest that they developed specific acquired immunity, with both T and B cells playing a role. Patients who recovered from COVID19 had elevated levels of neutralizing antibodies, and those who had longer illnesses had lower levels of neutralizing antibodies compared to those with shorter illness durations. T and B cell responses against COVID-19 are detectable in the blood about one week after the onset of symptoms. CD8+ T cells directly attack and kill virus-infected cells, while CD4+ T cells prime both CD8+ T cells and B cells and produce cytokines to recruit immune cells. The first autopsy of a COVID-19 patient showed an accumulation of mononuclear cells (likely monocytes and T cells) in the lungs and low levels of hyperactive T cells in the peripheral blood. These findings suggest that T cells are attracted to the infected site to control the viral infection. In COVID-19 patients, increased T cell exhaustion and reduced functional diversity predicted severe disease. Despite impaired response, patients who recovered from SARS-CoV infection developed coronavirus-specific memory T cells that were present at least two years after recovery. Cytotoxic T-cells and natural killer cells are crucial for generating an effective immune response against viruses [22], and functional exhaustion of these cells leads to disease progression [23]. Patients with COVID-19 had lower lymphocyte and higher neutrophil counts in blood compared to healthy controls. CD8+ lymphocytes and NK cells were significantly reduced in severe infections compared to patients with mild infections and healthy controls [21]. Our study found that the control of the virus infection by mouse anti-COVID-19 antiserum was lower when neutralization titers against COVID-19 were transferred into recipient mice. This suggests that the anti-infective activity of a neutralizing antibody is primarily mediated by preventing COVID-19 invasion, and the neutralizing antibody plays a lesser role in eliminating the virus after establishment of infection [32]. Therefore, we focused on the cooperation between anti-COVID-19 antibodies and other effectors in controlling COVID-19 infection. Potential effectors include complement (e.g. C3 and other complement-antibody complex pathway members), NK cells (mediators of Ab-dependent cellmediated cytotoxicity pathway), and Fc gamma receptor-bearing cells (e.g. alveolar macrophages, monocytes-derived infiltrating macrophages, and neutrophils). We used anti-Gr-1 and anti-Ly-6G antibodies to differentiate between monocytes and neutrophils. We tested the role of these effectors by selectively depleting them in a mouse infection model using CVF (complement depletion), antiIL-2Rβ antibody (NK cell depletion), clodronate liposomes (alveolar macrophage depletion), antiGr-1 antibody (monocytes/neutrophil depletion), or anti-Ly6G antibody (neutrophil depletion) before or after COVID-19 infection. The groups treated with clodronate liposome or anti-Gr-1 antibody, but not anti-Ly-6G antibody, failed to eliminate COVID-19 from their lungs by 9 days post-infection. Our results indicate that phagocytic cells such as monocyte-derived infiltrating macroph are crucial for effective clearance of
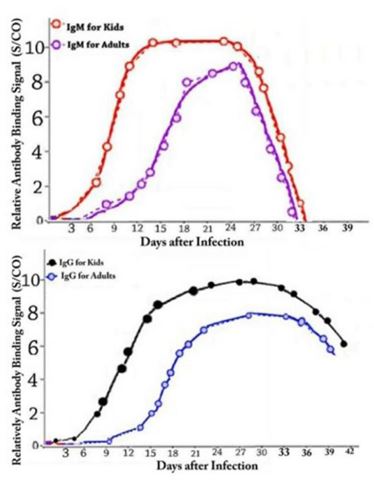
Figure 6: Average IgM and IgG in kids (13 kids in ages between 4 and 10) and adults patients (33 adults between ages 18 and 67) blood per days after infection to COVID-19. One may see IgM and IgG in kids patients are much higher than adult's patients in beginning days of infection but it becomes equal almost after 30 days. Higher IgM and IgG in beginning days’ cause production of much more natural antibodies in kids' bodies to protect them against COVID-19. This clearly shows why kids are more immune against COVID-19. It gives also idea about novel vaccination and medicine strategies to alleviate COVID-19.
COVID-19 from the lungs. These findings demonstrate the importance of evaluating the interplay between anti-COVID-19 Abs and other effectors in controlling viral infection. In conclusion, the outbreak of COVID-19 has resulted in a global health crisis with significant impact on mortality. The severity of the symptoms and mortality correlated with age-dependent defects of the immune response. The immune response to COVID-19 has been shown to involve both T and B cell responses, with T cells playing a crucial role in controlling the viral infection [27]. The results of this study have highlighted the importance of evaluating the interplay between anti- COVID-19 Abs and other effectors in controlling COVID-19 infection. Further research is needed to fully understand the mechanisms of viral clearance and to develop effective treatments for COVID-19. We investigated the innate and adaptive immune systems in human patients with COVID-19. In the early stages of infection, we found that natural antibodies play a crucial role. These antibodies, mostly of IgM and IgG isotypes, are produced independently of prior antigen encounters and have broad reactivity and variable affinity. In children, natural antibodies are produced by innate or IgM, IgG MBCs and are more abundant, explaining their greater immunity to COVID-19. Our findings show that the cooperation of antigen-specific antibodies and phagocytic cells (monocyte-derived infiltrating macrophages and partially alveolar macrophages) is crucial in controlling COVID-19 infection in both mouse models and human patients. This understanding can aid in the development of novel treatments for COVID-19. In COVID-19 patients, we observed a dramatic increase in CD4 γδ T cells within the γδ T cell population, while CD8 γδ T cells remained unchanged. This suggests that CD4 γδ T cells may play a role in antigen presentation and activation of adaptive immune cells during COVID-19 infection and serve as a bridge between innate and adaptive immunity. γδ T cells exhibit a strong activation phenotype in COVID-19 patients, as evidenced by increased CD25 expression. However, early activation marker CD69 showed no difference between patients and healthy donors. The expression of PD-1 did not differ between the two groups, suggesting that γδ T cells do not undergo exhaustion. In conclusion, γδ T cells are able to quickly respond to SARSCoV-2 infection and upregulate the activation marker CD25. They may work in parallel with other innate cells to defend against COVID-19. The increase in CD4 expression in γδ T cells may serve as a biomarker for COVID19 assessment.
Conclusion
For a vaccine to be effective, the body needs two types of immune cells - B cells and T helper cells - to produce antibodies. B cells are responsible for producing antibodies, while T helper cells improve the accuracy and strength of the antibodies. Identifying these helper immune cells could aid in future vaccine design, especially for vulnerable populations. T cells, along with antibodies, play a crucial role in the human immune response against viral infections by directly targeting and killing infected cells. In people who have recovered from COVID-19, T cell immunity was observed while antibodies disappeared 8-10 weeks after recovery. The level of antibodies in the blood is usually an indicator of vaccine efficacy, however, in the case of COVID-19, this is not accurate as antibodies disappear after a few weeks. To enhance immunity against COVID-19 in kids and adults, the first step is to produce phagocytes (IgM and IgG) for initial protection through innate immunity. Then, the production of γδT cells may act as a bridge between innate and adaptive immunity. A small portion of long-lived T cells, known as memory T cells, remains to provide rapid response upon re-exposure to the pathogen. Memory T cells have been trained to recognize specific antigens and will trigger a stronger and faster immune response upon re-encountering the same antigen. B cells are activated through T cells and contribute to protection through the production of antibodies. Memory T cells will protect recovered or vaccinated individuals from future infections. In most infections, the number of γδ T cells increases both locally and systemically a few days after infection. However, in the early stages of COVID-19, a decrease of γδ T cells was observed in symptomatic patients, suggesting a crucial rolefor these cells in the host immune response. Antibodies play a key role in the presence of phagocytes, but have a lesser role otherwise. Antibodies will disappear from the body of recovered patients after 8-10 weeks and should be considered when developing vaccines. However, memory T cells remain in the body for a longer time and this is promising for vaccine development. The main focus for COVID-19 research should be to boost the innate immune system, specifically phagocytes, which is the reason why children have better immunity against COVID-19. T cells play a crucial role in vaccine development, while the role of antibodies is not as critical as they disappear from the blood of recovered patients after 2-3 months.
Conclusion and Suggestion
Based on the results of this paper, we suggest the development of a vaccine against SARS-CoV-2 that elicits a protective immune response. This vaccine should utilize engineered peptides that strongly bind to the spike protein of the virus, and use these peptides to trigger the breakdown of viral proteins within cells [29, 30]. This type of vaccine shouldn't cause a cytokine storm [33]. The mRNA in the vaccine should encode a stable form of the spike protein, which will be processed by immune cells in the lymph nodes and recognized by other immune cells. The activation of T cells through MHC class-II will help compensate for the short-lived antibodies produced by the SARS-CoV-2 virus and provide long-term immunity through memory T cells. This vaccine approach, which combines peptide-encoding mRNA and T cell activation, is a relatively new genetic method that does not require the growth of the virus in a laboratory. Instead, it transforms the human body into a "living laboratory."
References
- Gorbalenya AE, Baker SC, Baric RS. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020; 5: 536544.
- Zhou P, Yang X, Wang X, Hu B, Zhang L. Addendum: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 588(7836): E6.
- Arvin AM. Varicella-Zoster virus: pathogenesis, immunity, and clinical management in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2000; 6(3): 219-230.
- Nagel MA, Cohrs RJ, Mahalingam R, Wellish MC, Forghani B. The varicella zoster virus vasculopathies: Clinical, CSF, imaging, and virologic features. Neurology. 2008; 70(11): 853-860.
- Mueller NH, Gilden DH, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus infection: clinical features, molecular pathogenesis of disease, and latency. Neurol Clin. 2008; 26(3): 675697.
- Ragozzino MW, Melton3rd LJ, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore). 1982; 61(5):310-316.
- Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005; 20(8): 748-753.
- Chuan Qin, Luoqi Zhou, Ziwei Hu, Zhang S, Yang S. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020; 71(15): 762768.
- Roberts A, Paddock C, Vogel L, Butler E, Zaki S. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J Virol. 2005; 79(9): 5833-5838.
- Roberts A, Deming D, Paddock CD, Cheng A, Yount B. A mouse-adapted SARS- coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007; 3(1): e5.
- Nagata N, Iwata N, Hasegawa H, Fukushi S, Harashima A. Mousepassaged severe acute respiratory syndrome-associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. Am J Pathol. 2008; 172(6): 1625-1637.
- Zhao J, Perlman S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J Virol. 2010; 84(18): 9318-9325.
- Zhao J, Van Rooijen N, Perlman S. Evasion by stealth: inefficient immune activation underlies poor T cell response and severe disease in SARS-CoV-infected mice. PLoS Pathog. 2009; 5(10): e1000636.
- Chen J, Lau YF, Lamirande EW, Paddock CD, Bartlett JH. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010; 84(3): 1289-1301.
- Yasui F, Kohara M, Kitakabe M, Nishiwaki T, Fujii H. Phagocytic cells contribute to the antibody-mediated elimination of pulmonary-infected SARS coronavirus. Virology. 2014; 454455:157168.
- J. Zhao, S. PerlmanT cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected miceJ. Virol. 2010; 84(18): 9318-9325.
- Tanaka T, Kitamura F, Nagasaka Y, Kuida K, Suwa H. Selective longterm elimination of natural killer cells in vivo by an anti-interleukin 2 receptor beta chain monoclonal antibody in mice. J Exp Med. 1993; 178(3): 1103-1107.
- Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST. Lung pathology of fatal severe acute Respiratory Syndrome. Lancet. 2003; 361(9371): 1773-1778.
- Pribul PK, Harker J, Wang B, Wang H, Tregoning JS. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J Virol. 2008; 82(9): 4441-4448.
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 83(1): 64-70.
- Huang C, Wang Y, Li X, Ren L, Zhao J. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395(10223): 497-506.
- Chen N, Zhou M, Dong X, Qu J, Gong F. Epidemiological and clinical characteristics of 99cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395(10223): 507-513.
- Zheng M, Gao Y, Wang G, Song G, Siyu L. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17(5): 533-535.
- Zhao J, Yuan Q, Wang H, Liu W, Liao X. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. ClinInfect Dis. 2020.
- Zhao J, Yuan Q, Wang H, Liu W, Liao X. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Journal of allergy and Clinical Immunology 2020.
- Long Q-X, Liu B-Z, Deng H-J. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nature Medicine. 2020; 26: 845-848.
- Sun B, Feng Y, Mo X, Zheng P, Wang Q. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020; 9(1): 940-948.
- Farshi E, Immunological Reason for Mild Affection of Children to COVID-19, A Key Factor for Novel Solution of Vaccination and Medications. Japanese J Gstro Hepato. 2021; V8(2): 1-9
- Farshi E. Peptide-Based mRNA Vaccines. J Gastro Hepato.2023; V9(16): 1-6
- Farshi E. Peptide-mRNFarshi Vaccine for SARS-Cov-2. J Vaccines Vaccin. 2020; 11: 431.
- Farshi, E. “Simulation of Herd Immunity in Covid-19 Using Monte Carlo Method.” Austin J Pulm Respir Med 7.1 (2020): 1066.
- Farshi, Esmaeil, Bahram Kasmapur, and Anya Arad. “Investigation of immune cells on elimination of pulmonary-Infected COVID-19 and important role of innate immunity,phagocytes.” Reviews in Medical Virology 31.2 (2021): e2158.
- Farshi, Esmaeil. “Cytokine storm response to COVID-19 vaccinations.” J Cytokine Biol 5.1000125 (2020): 2.
- Bonifacius, Agnes, et al. “COVID-19 immune signatures reveal stable antiviral T cell function despite declining humoral responses.” Immunity 54.2 (2021): 340-354.
- Xu W, Lau ZWX, Fulop T, Larbi A. The Aging of γδ T Cells. Cells. 2020; 9: 1181.

