Case report - Volume 3 - Issue 2
Concomitant Occurrence of Non-cirrhotic Portal Hypertension, Multiple Acute Cerebral Infarcts, and Lupus Nephritis in a Patient with Systemic Lupus Erythematosus
Maolu T1,5; Rui X2,; Jing Y1; Hong P3; Yan Z1,4,5*
1Department of Nephrology, Institute of Nephritic and Urinary Disease, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China.
2Department of Radiology, Guizhou Provincial People’s Hospital, Guiyang, China.
3Infectious Diseases Department, Guizhou Provincial People’s Hospital, Guiyang, China.
4Department of Pulmonary Medicine, NHC Key Laboratory of Pulmonary Immunological Diseases, Guizhou Provincial People’s Hospital, Guiyang, China.
5Guizhou University medical college, Guiyang, China.
*Maolu Tian and Rui Xu contributed equally to this work and share first authorship.
Received Date : Feb 20, 2023
Accepted Date : Mar 23, 2023
Published Date: Mar 30, 2023
Copyright:© Yan Z 2023
*Corresponding Author : Yan Z, Department of Nephrology, Institute of Nephritic and Urinary Disease, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China.
Email: zhayansy@163.com
DOI: Doi.org/10.55920/2771-019X/1406
Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease that is characterized by multiple organ involvement with extremely variable and heterogeneous clinical presentations. Patients with SLE do not often experience multiple acute ischemic strokes, and liver involvement caused by SLE itself, such as cirrhosis, portal hypertension, or hepatic encephalopathy, is even rarer. In this paper, we report on a 35-year-old woman with SLE simultaneously presenting with multiple acute ischemic strokes, portal hypertension, nodular regenerative hyperplasia and lupus nephritis. To our knowledge, such a concomitant occurrence has rarely been reported in SLE patients before.
Keywords:
Systemic lupus erythematosus; acute cerebral infarction; portal hypertension; nodular regenerative hyperplasia; lupus nephritis
Case presentation
On April 05, 2019, a 35-year-old woman, who had had hypertension for 1 year, was admitted to the Infectious Diseases Department of Guizhou Provincial People's Hospital due to abdominal distension, abdominal pain and edema in the lower extremities (which had been occurring for more than one month), and melaena (which had been occurring for 2 days). Before admission, the patient had recently undergone gastroscopic examination at a county-level hospital, which showed esophageal varices presenting as positive 'red signs', and chronic non-atrophic gastritis with remote hemorrhaging. Upper abdominal computed tomography (CT) examination at the same county-level hospital revealed cirrhosis, splenomegaly and ascites. She had no history of drug abuse or significant alcohol consumption prior to admission. Physical examination on admission showed hepatosplenomegaly and symmetric pitting edema of both lower extremities, but the abdominal wall veins were not obviously dilated. Malar rash, enlarged parotid glands and arthritis were absent. Her blood pressure was 130/80 mmHg. The antibodies that indicate autoimmune hepatitis, hepatitis virus, tumor markers and ceruloplasmin were all negative. Tests for ascites were conducted in triplicate and no obvious abnormalities were found in the biochemistry, pathogen culture or tumor markers. A CT scan of the chest showed small amounts of bilateral pleural effusion, a small amount of pericardial effusion, bilateral pneumonic lesions, and multiple lymph nodes in the bilateral axilla. A plain film of the abdomen showed no signs of ileus. Both abdominal ultrasonography (USG) and enhanced upper abdominal CT showed cirrhosis-like nodular regenerative hyperplasia, hepatosplenomegaly and ascites, without any obvious signs of portal vein dilation or thrombosis (Fig. 1). In addition, varices of the esophagus and fundus were found in the enhanced upper abdominal CT (Fig. 1), along with swollen small intestine walls. A transthoracic echocardiogram revealed enlargement of the right atrium and right ventricle, moderate tricuspid regurgitation, mild mitral and aortic regurgitation, moderate pulmonary hypertension, and normal left ventricular systolic function.
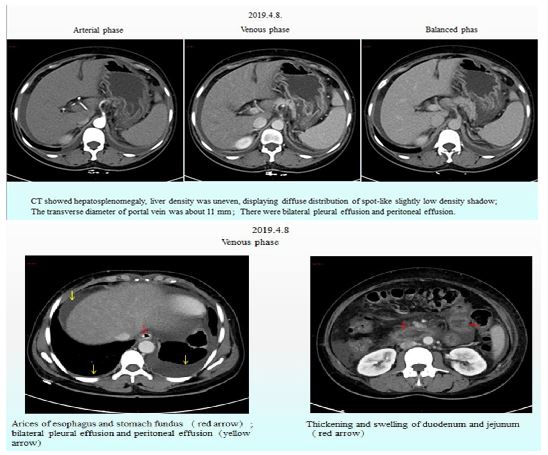
Figure 1: Enhanced upper abdominal CT.
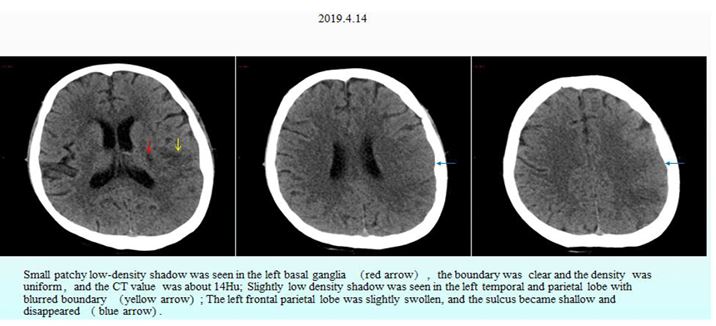
Figure 2: Nonenhanced CT of the head.
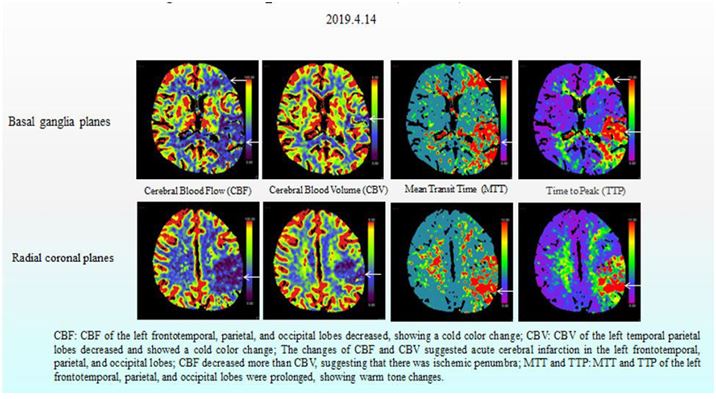
Figure 3: CT perfusion (CTP) of the head.
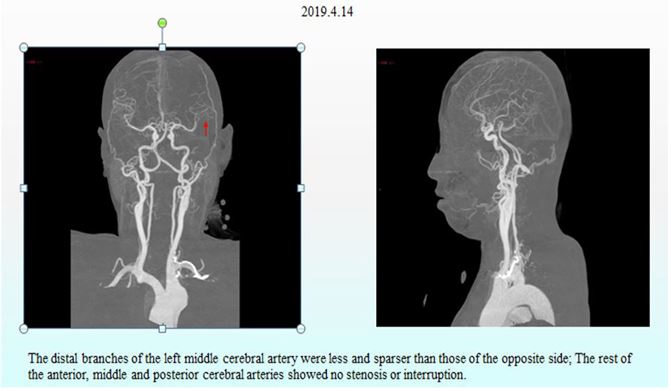
Figure 4: CT angiography (CTA) of the head and neck.
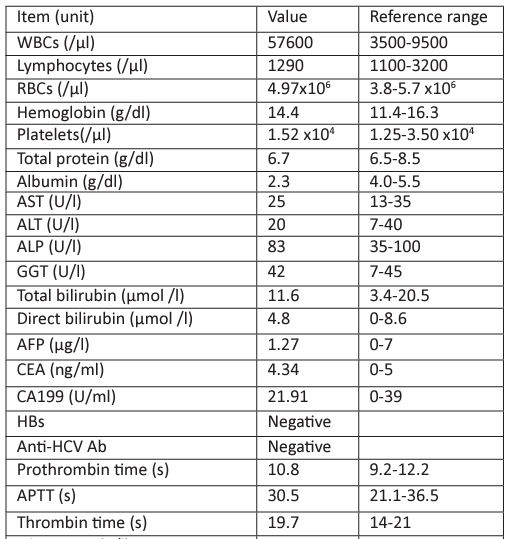
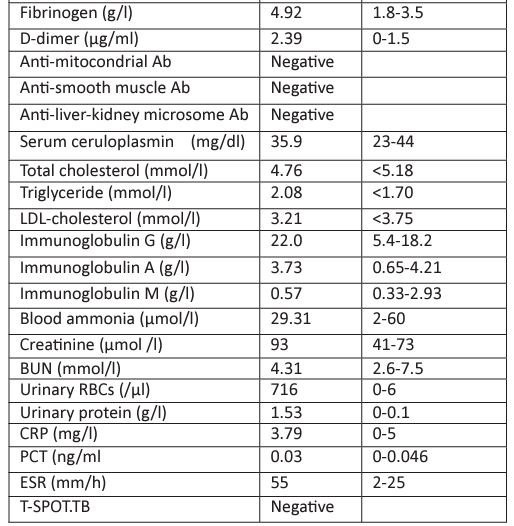
Laboratory results for tests conducted after the patient was admitted are shown in Table 1. Our department was invited to consult this patient because of obvious proteinuria and microscopic hematuria. The patient was interviewed and a detailed medical history was obtained. We learned that the patient had experienced obvious alopecia, hyperphotosensitivity, Raynaud's phenomenon and mild dry mouth in the past six months. Therefore, multiple autoantibodies associated with autoimmune diseases such as antinuclear antibody profile and anti-neutrophilic cytoplasmic antibody profile were requested to be examined. Levels of serum complements, including C3 and C4, were also requested.
After the patient received some routine treatment, such as inhibiting gastric acid secretion to protect the stomach and liver, reducing portal pressure, and controlling blood pressure, the symptoms that the patient had been experiencing during admission were somewhat relieved. However, the patient had a sudden disturbance of consciousness at around 3:00 am on April 15, 2019, manifesting as agitation, aphasia, lingual paralysis on the right side, and weakness of the right limb. The day after onset of these symptoms, non-enhanced CT and CT perfusion (CTP) of the head were performed, along with CT angiography (CTA) of the head and neck. These cranial imaging examinations revealed multiple acute cerebral infarctions in the left cerebral hemisphere due to thrombosis and occlusion of the distal left middle cerebral artery (Fig. 2, Fig. 3 and Fig. 4).
By that time, the results of the autoantibody panel test and serum complements were also available, showing a positive result for the antinuclear antibody (ANA 1:1000). Anti-SS-A/SS-B/Ro52 antibodies were all strongly positive, but the anti-ribosomal P antibody was negative. The anti-neutrophil cytoplasmic antibody, anti-glomerular basement membrane antibody and anticardiolipin antibody tests (IgG and IgM) were negative. The levels of serum complements including C3 (0.58 g/L; normal 0.82-1.93 g/L), and C4 (0.12 g/L; normal 0.15-0.57 g/L) were reduced. Moreover, the patient’s serum creatinine increased to 263 umol/L.
A diagnosis of SLE with multiple organ involvement was made based on the above symptoms, signs and examination results. Considering the severity of the disease activity, the patient was treated according to the SLE management guideline published by European League Against Rheumatism (EULAR) [1], with intravenous methylprednisolone 500 mg for 1 day, 800 mg for 2 days and then 40 mg/day. This high dose glucocorticoid regimen was given once again ten days later. Cyclophosphamide, hydroxychloroquine and plasma exchange were also administered to this patient. High resolution MR imaging of the cerebral artery wall on April 23, 2019 revealed abnormal thickening of multiple bilateral intracranial vessel walls, which was considered as vasculitis (Fig. 5). Biopsy of the labial gland revealed dilated salivary ducts and mildly focal infiltration of lymphocytes (ChisholmⅠlevel) between acini. The study protocol was approved by the ethics committee of Guizhou Provincial People’s Hospital and conducted in adherence to the ethical guidelines stated in the Declaration of Helsinki. Written informed consent was obtained from this patient before her enrollment.
After active immunosuppression and symptomatic treatment, the patient's condition improved significantly. The patient regained muscle strength in her right limb and she could walk with help. Although the patient still could not communicate with normal language, she could produce ambiguous speech without headache, irritability or delirium. The patient did not have abdominal pain, abdominal distension or melaena, and the edema in both lower extremities decreased. Laboratory results confirmed that the serum albumin (30.4 g/l) had increased, the renal function returned to normal, the liver function and blood routine showed no abnormalities, the fecal occult blood test was negative, and pleural and abdominal fluid had decreased. Cranial MR scan and diffusion-weighted imaging (DWI) were performed on April 19 and May 4, 2019, respectively. Compared with the scan performed on April 19, the cerebral infarction lesion range had decreased significantly, and the degree of diffusion limitation was reduced (Fig. 6). Unfortunately, during the telephone follow-up, we learned that the patient had died within six months after discharge.
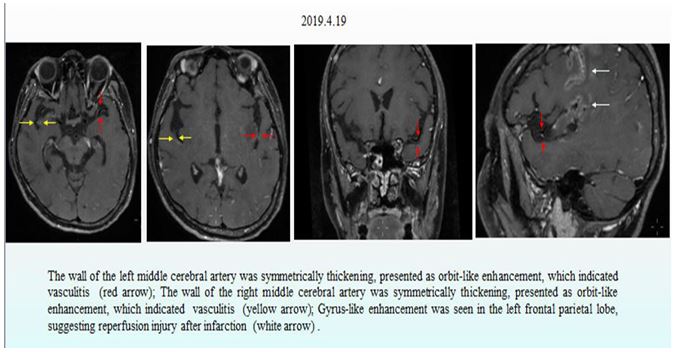
Figure 5: MR high resolution imaging of cerebral artery wall.
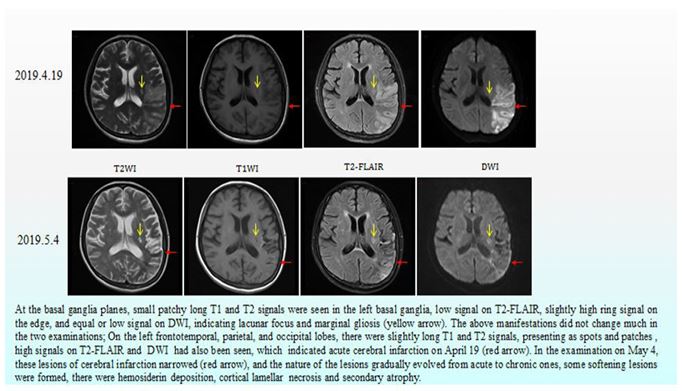
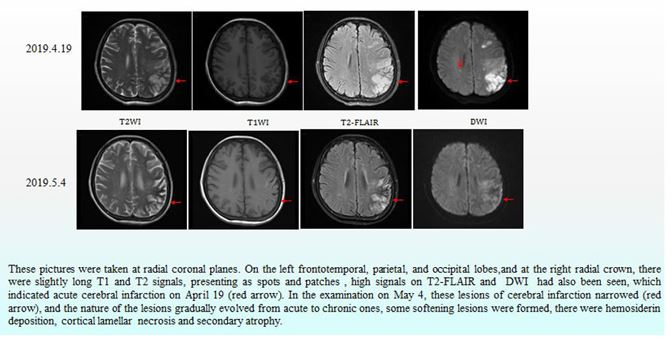
Figure 6: Cranial MR and diffusion-weighted imaging (DWI).
Discussion
Although a variety of organs can be involved in SLE, damage to digestive system organs is less common, especially liver damage. Hepatic involvement in SLE can be classified as primary (SLE related) and secondary (non-SLE related). Secondary antiphospholipid syndrome (APS), secondary Sjogren's syndrome, Raynaud's phenomenon or anti-ribosomal P antibodies have been found to be associated with the development of SLE-related liver damage [2-4]. Non-SLE related hepatic involvement in patients with SLE is usually a result of drug-induced liver injury, fatty liver, viral hepatitis, or rarely due to an overlapping autoimmune liver disease. After exclusion of secondary causes, hepatitis due to SLE may be seen in 3%–5% of cases [5]. SLE-related hepatic abnormalities are usually synchronous with disease activity [6]. A recent study by Zheng et al [7] based on their strict discrimination criteria reported a 9.3% incidence of lupus hepatitis among the 504 SLE patients that were evaluated. They also reported that the prevalence of lupus hepatitis in patients with active SLE was higher than those with inactive SLE (11.8% vs 3.2%) [7]. The patients with hepatic involvement due to SLE mostly showed mild to moderate elevations of serum transaminase levels. Large multicenter studies of mortality in SLE have shown that hepatitis was not a major cause of morbidity or mortality [8, 9]. Therefore, even the latest SLE classification criteria published in 2019 by EULAR and the American College of Rheumatology (ACR) [10] did not consider hepatic involvement relevant for establishing a diagnosis of SLE, and none of the measurement tools included any liver criteria to evaluate disease activity. This exclusion was probably due to the rarity of primary hepatic involvement and the benign, transient course of these abnormalities [11].
However, in recent years, there have been some reported cases of severe liver damage due to SLE, such as non-cirrhotic portal hypertension (NCPH), cirrhosis and even liver failure [12]. According to the literature search by Yang QB et al., 22 cases of NCPH associated with SLE were reported from 1994 to 2017 [13]. Pulmonary arterial hypertension (PAH) and pancytopenia (PCP) were also observed in 18.2% (4/22) of patients. Portal thrombosis was not detected in any of the patients. Ten (45.5%) of those patients did not have hepatic dysfunction according to clinical laboratory tests. Furthermore, the majority of patients had nodular regenerative hyperplasia (NRH, 16/22), shown by hepatic histopathology. NRH usually presents with clinical symptoms of NCPH with normal liver function tests, and imaging studies could support a diagnosis, but it is confirmed definitively with a liver biopsy and histopathological findings. HU Rong-rong et al. [14] summarized retrospectively the clinical data of 12 SLE-NCPH patients admitted to Peking Union Medical College Hospital between 1996 to 2013, and they reported no neuropsychiatric complications in the NCPH-SLE group, while thrombocytopenia in the NCPH-SLE group occurred more frequently than in the control group. HU Rong-rong et al. [14] also found that the effects of steroids and immunosuppressive agents had minimal effect in improving portal hypertension (PHT). The prognosis of SLE patients was affected by complications of portal hypertension and PHT management may have been helpful in relieving their symptoms. In 2020, You H et al. [15] conducted a case-control study, and a total of 21 patients with SLE cirrhosis were enrolled. You H et al. [15] found that cirrhosis was a rare and severe subtype of SLE with a poor prognosis, and more attention should be paid to those patients with hematological system involvement and impaired liver function.
The exact pathogenesis of SLE-related liver damage, especially NCPH and cirrhosis remains unclear. The deposition of immune complex and complement in the vascular wall might cause vasculitis in the liver, lumen partial or complete occlusion leading to liver cell necrosis and regeneration, followed by the formation of NRH and NCPH which eventually develops into cirrhosis [16, 17]. Vasospasms caused by Raynaud's phenomenon, microthrombosis caused by coagulation disorders with positive anti-phospholipid antibodies, and some immunosuppressants (cyclophosphamide, azathiopine) might be high risk factors for the development of NCPH [17].
This patient had a short medical history and no history of drug abuse or alcohol addiction. Routine blood tests did not show any abnormalities. There was no increase in blood ammonia or decrease in coagulation function (instead, hypercoagulability was found), and liver function indexes were within the normal range. Sjogren's syndrome, Raynaud's phenomenon and pulmonary hypertension were secondary to SLE. Other hepatic diseases such as viral hepatitis and hepatolenticular degeneration were excluded based on the patient’s laboratory results and medical history. Therefore, the patient was diagnosed with active SLE complicated by SLE related NCPH according to her clinical features and auxiliary investigations [18], despite the lack of liver histopathology due to the patient’s refusal for a liver biopsy. Of note, the diagnosis of NCPH was also supported by the patient’s response to treatment.
The diagnostic criteria for neuropsychiatric systemic lupus erythematosus (NPSLE) was proposed by the ACR in 1999, including 19 neurological syndromes (12 central and 7 peripheral NP) [19]. NPSLE is one of the most serious disorders in SLE and is usually associated with a poor prognosis. It has been reported that an acute confused state, seizures, and cerebrovascular disease in NPSLE were risk factors that affected survival [20]. The incidence of NPSLE ranged from 12.2% to 94.7% for SLE patients [21, 22], and stroke was reported to occur in 3%–30% of patients with SLE, most of which were ischemic strokes [23]. The diversity and heterogeneity of NP presentations suggested that multiple pathogenetic mechanisms such as antibodies, cell-related inflammation, cytokine-related inflammation, complement activity and the presence of secondary APS were involved in NPSLE [24,25]. Previous studies showed that NPSLE usually occurred within 3 years after onset of SLE [25, 26], and high disease activity was likely associated with diffuse NP manifestations of the central nervous system [27]. However, NPSLE may precede lupus or may occur during latent phase in some patients, which may be related to the regional immune inflammation of the nervous system.
Cranial magnetic resonance imaging (MRI) is still the most commonly used imaging technique for detecting and evaluating NPSLE in clinical practice. Abnormal cranial MRI results were divided into inflammatory lesions, small vessel disease (SVD), and large vessel disease (LVD) [25]. The prevalence of inflammatory lesions ranged from 0% to 38.1% in patients who underwent MRI [28, 29]. A previous study showed that inflammatory lesions might be related to cerebral vasculitis in SLE patients, which can be diffuse or have focal distribution, involving both the large and small arteries [30]. According to the standards for reporting vascular changes observed on neuroimaging, SVD was divided into white matter hyperintensity, subcutaneous small infarcts, luminal infarction, microbleeding, and brain atrophy [31]. LVD, defined as cerebral infarction in the great arteries area, was described according to the number of lesions (single/multiple), related arteries, and status (acute/chronic) [25]. Patients with inflammatory lesions and LVD on MRI may have poorer prognoses [25].
In this young woman, NP events caused by other diseases such as cardiogenic thromboembolism, central nervous system infections, metabolic abnormalities, neoplasm, trauma, electrolyte imbalance and toxic exposure were excluded. Active SLE was considered to be the cause of multiple acute cerebral infarctions, and immunosuppressive therapy for NPSLE was effective in improving her brain lesions. Vasculitis, which was suggested by imaging, might be the possible cause of her acute cerebral infarctions. The APS related antibody spectrum was not extensively tested for in this patient, so secondary APS could not be completely excluded.
Table 1 : Laboratory parameters at admission.
This was a comparatively rare case that deserves to be reported. The patient was predicted to have an unfavorable prognosis, given the fact that NCPH, cerebrovascular disease, and lupus nephritis had coexisted in this patient, and that she had poor compliance. Actually, the patient died within six months after disease onset.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- Fanouriakis A, Kostopoulou M, Alunno A. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736-745.
- Ohira H, Takiguchi J, Rai T, Abe K, Yokokawa J, Sato Y, Takeda I, Kanno T. High frequency of anti-ribosomal P antibody in patients with systemic lupus erythematosus-associated hepatitis. Hepatol Res 2004; 28: 137-139.
- Luangjaru S, Kullavanijaya P. Gastrointestinal and hepatobiliary manifestations in systemic lupus erythematosus. J Med Assoc Thai 2005; 88: 71-75.
- Shizuma T. Clinical Characteristics of Concomitant Systemic Lupus Erythematosus and Primary Biliary Cirrhosis: A Literature Review. J Immunol Res. 2015;2015:713728.
- Arnett FC, Reichlin M. Lupus hepatitis: an under-recognized disease feature associated with autoantibodies to ribosomal P. Am J Med 1995;99:465-72.
- González-Regueiro JA, Cruz-Contreras M, Merayo-Chalico J. Hepatic manifestations in systemic lupus erythematosus. Lupus. 2020;29:813-824.
- Zheng RH, Wang JH, Wang SB, Chen J, Guan WM, Chen MH. Clinical and immunopathological features of patients with lupus hepatitis. Chin Med J (Engl) 2013; 126: 260-266.
- Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Medicine Baltimore 2006;85:147-56.
- Mok CC, Mak A, Chu WP, To CH, Wong SN. Long-term survival of southern Chinese patients with systemic lupus erythematosus: a prospective study of all age-groups. Medicine Baltimore 2005;84:218-24.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019; 78:1151-1159.
- Romero-Diaz J, Isenberg D, Ramsey-Goldman R. Measures of adult systemic lupus erythematosus: updated version of British Isles Lupus Assessment Group (BILAG 2004), European Consensus Lupus Activity Measurements (ECLAM), Systemic Lupus Activity Measure, Revised (SLAM-R), Systemic Lupus Activity Questionnaire for Population Studies (SLAQ), Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), and Systemic Lupus International
- Collaborating Clinics/American College of Rheumatology Damage Index (SDI). Arthritis Care Res 2011; 63: S37-S46.
- Bessone F, Poles N, Roma MG. Challenge of liver disease in systemic lupus erythematosus: Clues for diagnosis and hints for pathogenesis. World J Hepatol. 2014;6:394-409.
- Yang QB, He YL, Peng CM, Qing YF, He Q, Zhou JG. Systemic lupus erythematosus complicated by noncirrhotic portal hypertension: A case report and review of literature. World J Clin Cases. 2018;6:688-693.
- Hu, S. Zhang, M. Li, Y. Zhao, F. Zhang, X. Zeng. Clincal characteristics of systemic lupus erythematosus with non-cirrhotic portal hypertension . Chin J Allergy Clin Immunol. 2018:12:289-295.
- You H, Peng L, Zhao J, Fei Y, Wang Q, Zhang W, Li M, Zeng X. Clinical Characteristics of Systemic Lupus Erythematosus with Cirrhosis. J Immunol Res.2020;2020:2156762.
- Matsumoto, T. Yoshimine, K. Shimouchi, H Shiotu, N Kuwabara, Y Fukuda, T Hoshi. The liver in systemic lupus erythematosus: pathologic analysis of 52 cases and review of Japanese autopsy registry data. Human Pathology.1992;23:1151-1158.
- Zhang, H. Liu, H. Yao, Y. Jia, Z. Li. Systemic lupus erythematosus complicated by noncirrhotic portal hypertention: a clinical analysis and review of literature. Chin J Rheumatol. 2017; 21: 327-332.
- Sarin, SR. Aggarwal. Idiopathic portal hypertension. Digestion. 1998; 59: 420-423.
- ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis & Rheumatism.1999; 42:599-608.
- Tokano, S. Morimoto, H. Amano, T. Kawanishi, T. Yano, M. Tomyo, M. Sugawara, S. Kobayashi, H. Tsuda, Y. Takasaki, H. Hashimoto. The relationship between initial clinical manifestation and long-term prognosis of patients with systemic lupus erythematosus. Modern Rheumatology. 2005; 15:275-282.
- Unterman, J. E. Nolte, M. Boaz, M. Abady, Y. Shoenfeld, and G. Zandman-Goddard. Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Seminars in Arthritis and Rheumatism. 2011: 41:1-11.
- Petri, A. M. Orbai, G. S. Alarcón et al., Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis & Rheumatism. 2012; 64:2677-2686.
- So-Yeon Kim, Tae-Sik Yoon, Jee-Hyun Suh. Concomitant Occurrence of Cervical Myelopathy, Cerebral Infarction, and Peripheral Neuropathy in Systemic Lupus Erythematosus: A Case Report. Ann Rehabil Med 2014;38:263-268.
- Schwartz, A. D. Stock, and C. Putterman. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nature Reviews Rheumatology. 2019; 15:137-152.
- Zhang S, Li M, Zhang L, Wang Z, Wang Q, You H, Wang Y, Li M, Zeng X. Clinical Features and Outcomes of Neuropsychiatric Systemic Lupus Erythematosus in China. J Immunol Res. 2021;2021:1349042.
- Li, X. Xiang, J. Sun, S. Liu, Y. Liu, L. Feng, C. Li, Z. Li. Prevalence, outcome and prognostic factors of neuropsychiatric systemic lupus erythematosus: a real world single center study. Modern rheumatology. 2020; 30:321-326.
- G. Hanly and M. J. Harrison. Management of neuropsychiatric lupus. Best Practice & Research Clinical Rheumatology. 2005; 19:799-821.
- Toledano, N. Sarbu, G. Espinosa, N. Bargalló, and R. Cervera. Neuropsychiatric systemic lupus erythematosus: magnetic resonance imaging findings and correlation with clinical and immunological features. Autoimmunity Reviews. 2013;12:1166-1170.
- Tan, Y. Zhou, X. Li, G. Wang, J. Tao, L. Wang, Y. Ma, X. Li. Brain magnetic resonance imaging, cerebrospinal fluid, and autoantibody profile in 118 patients with neuropsychiatric lupus. Clinical Rheumatology. 2018; 37: 227-233.
- G. Jennekens and L. Kater. The central nervous system in systemic lupus erythematosus. Part 2. Pathogenetic mechanisms of clinical syndromes: a literature investigation. Rheumatology. 2002; 41:619-630.
- M. Wardlaw, E. E. Smith, G. J. Biessels et al., Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology. 2013; 12: 822-838.

