Case report - Volume 3 - Issue 2
Acute Painful Diabetic Neuropathy in two newly diagnosed adolescents with Type 1 Diabetes and Eating Disorder
Maria K¹*(https://orcid.org/0000-0003-0226-9372); Marina K² (https://orcid.org/0000-0003-4996-5682); Irine-Ikbale S¹(https://orcid.org/0000-0003-3984-1790); Dimitra K¹(https://orcid.org/0000-0002-13129423); Maria L¹(https://orcid.org/0000-0003-2496-0244); Spyridon K¹(https://orcid.org/0000-0003-23101050); Kyriaki K¹(https://orcid.org/0000-0001-5323-2786)
1Diabetes and Metabolism Unit, 2nd Department of Pediatrics, National and Kapodistrian University of Athens, “P&A Kyriakou” Children’s Hospital, 11527 Athens, Greece.
2Neurology Department, “Aghia Sophia” Children’s Hospital, Athens, Greece.
Received Date : Feb 27, 2023
Accepted Date : Mar 30, 2023
Published Date: April 06, 2023
Copyright:© Maria K 2023
*Corresponding Author : Maria K, Diabetes and Metabolism Unit, 2nd Department of Pediatrics, National and Kapodistrian University of Athens, “P&A Kyriakou” Children’s Hospital, 11527 Athens, Greece.
Email: mka274274@gmail.com
DOI: Doi.org/10.55920/2771-019X/1411
Abstract
Type 1 Diabetes (T1D) is the most common type of diabetes among children and adolescents. The related chronic macrovascular and microvascular complications are associated with high long-term risk for morbidity and early mortality. We hereby present 2 cases of adolescents with newly diagnosed Type 1 Diabetes (9.8 months and 2 years, respectively) and Eating Disorder (ED) who developed Acute Painful Diabetic Neuropathy (APDN). Both patients had multiple risk factors for Acute Painful Diabetic Neuropathy that have attributed to the early appearance of the disease, such as acute deterioration of glycaemic control, rapid and extreme weight changes and poor compliance with insulin therapy. APDN is a rare Diabetes complication in youth that may appear after extreme weight changes usually in the context of ED. Poor glycemic control is a major risk factor for the development of APDN. Remission of symptoms may occur following restoration of optimal diabetic control and weight normalization. However, neuropathy symptoms may recur following future glycemic deterioration.
Key words : Type-1-Diabetes; Eating-Disorder; Acute-Painful-Diabetic-Neuropathy; Adolescence.
Introduction
Type 1 Diabetes (T1D) is the most common type of diabetes among children and adolescents, accounting worldwide for ≥ 85% of all diabetes cases in patients under 20 years old [1]. The related chronic macrovascular and microvascular complications are associated with high long-term risk for morbidity and early mortality. According to ADA Consensus Statement (San Antonio, 1988), Diabetic Neuropathy (DN) is "a descriptive term meaning a demonstrable disorder, either clinically or sub-clinically evident, that occurs in the setting of Diabetes Mellitus without other causes for peripheral neuropathy. The neuropathic disorder includes manifestations in the somatic and/or autonomic parts of the peripheral nervous system" [2]. Diabetic peripheral Neuropathy (DPN) is a common type of neuropathy as it affects 50% of the diabetic population.[3]. The clinical presence of DPN is defined by symptoms consistent with peripheral sensorimotor polyneuropathy, combined with either abnormal nerve conduction of at least two peripheral nerves or unequivocally abnormal autonomic neural tests [4, 5].
DPN is well met in the paediatric population, with a prevalence ranging between 7% to 62% among T1D children and adolescents [6, 7]. The main risk factors for the development of DPN are older age, long T1D-duration, smoking, high diastolic blood pressure, obesity, increased LDL-c and triglycerides, and lower HDL cholesterol [6]. Complications of DPN are chronic pain, foot ulceration and even amputation, with most existing treatments providing mainly symptomatic relief [8]. In agreement, Diabetes Control and Complications Trial (DCCT) advises that early optimal glycaemic control could delay clinically significant nerve impairment or even reverse the progression of DPN [9]. As such early detection of children and adolescents with DPN is of paramount importance in order to maintain an optimal glycaemic control [5, 9].
It is usually difficult to identify DPN in children as in most cases they are asymptomatic or present with non-specific symptoms mimicking other pathologies like chronic renal failure, vitamin deficiencies, malignancy, sickle cell disease, connective tissue disorders and enteropathy [10]. The proposed pathogenetic mechanisms of DPN include microvascular‐induced ischaemia, the production of extracellular advanced glycation end‐products (AGEs), inflammatory cytokines, increased aldose reductase activity and increased activation of the polyol pathway [11].
Acute painful diabetic neuropathy (APDN) is a rare subtype of painful diabetic neuropathy, first mentioned in 1974 by Ellenberg [12]. It is a small-fiber polyneuropathy that may present shortly after the diagnosis of diabetes, possibly precipitated by the initiation of insulin therapy, with symptoms which usually last less than 6 months. On the other hand, chronic Painful Neuropathy is a painful polyneuropathy, which usually starts later in the course of the disease and persists for more than 6 months [13].
Adolescents and especially females diagnosed with T1D peri-pubertally seem to be at greater risk of developing Eating Disorder (ED) compared to general population, which in turn may trigger or aggravate long term complications of DM such as Neuropathy or Nephropathy [14, 15]. In this report we present two adolescent T1D cases with early presentation of APDN. Both patients had concomitant ED associated with poor glycaemic control.
CASE A
An 11-years-old peripubertal girl was admitted to our hospital due to a severeDiabetic Ketoacidosis (DKA) episode accompanied by rapid weight loss (-3kg). The DKA episode (capillary Glucose 399mg/dl, pH 7.03, HC03 5 mEq/L) resolved with specialised medical management and the girl was diagnosed with T1D. The patient received in hospital intensive Diabetes management education and discharged 10 days later.
She previously was an average-weight pubertal girl (height 75th percentile, weight 25-50th percentile, BMI 10-25th percentile) according to published national charts and apart from mild hypercholesterolemia (conservative management since 4 years old) was otherwise healthy [16]. She was born at term (no history of maternal DM) and normal birth weight (3.560gr).
Although optimal metabolic control was achieved in the first 3 months (HbA1c 7.6%) after discharge, she progressively developed Eating Disorder triggered by high levels of psychological stress following T1D diagnosis. Consequently, her metabolic control deteriorated (HbA1c 8.5% and 11% at 6 and 9 months respectively) while lost a significant amount of weight [weight: 36.6 kg, (15th percentile)].
Eight months following T1D diagnosis, she presented with a second DKA episode (pH7.11, HCO3 8 mEq/L) as a result of poor glycaemic control, omission of fast rapid acting insulin and carbohydrate free diet (as a measure to control her weight) at least for 20 days, resulting in -2 kg extra weight loss [BMI :14.1kg/m2, (10th percentile)] and temporary cessation of menstruation. The girl was diagnosed with unspecified ED, as criteria for anorexia nervosa were not fulfilled according to DSM-V (Diagnostic and Statistical Manual of mental disorders 5th ed).
Although the DKA episode was initially resolved, the patient (48 hours after her admission) experienced numbness over her upper and lower extremities (Table 1). Nerve conduction studies (NCS) were performed by an experienced Neurologist (M.K.) and impaired vibration sensation threshold (VST) measurements were verified in the upper and lower extremities using a biothesiometer (Newbury, Oh). VST measurements were conducted bilaterally (Table 2), and the VST value for each site was calculated as the mean of three consequent measurements. Furthermore, peroneal, sural and median nerves of the left limbs were assessed by sensory and motor NCS. Abnormal values were recorded in the amplitude and conduction velocity of the sensory peroneal nerve, which according to published data [17], indicate axonal and demyelinating lesions in peripheral nerves (Table3). As a consequence of the patient's double ED/ DPN diagnosis, a multidisciplinary team consisting of a Pediatric Diabetologist, a clinical Dietician, a Paediatric Psychiatrist and a Neurologist closely monitored her progression. Under careful supervision she regained 5.4 kg and achieved optimal metabolic control (HbA1c 6.5%) in the next 6 months (Table 1). During these months the girl admitted sporadic binge eating episodes, but no purging behavior or insulin omission. Her neuropathic symptoms improved apart from rare episodes of numbness in fingertips, most often affecting her lower extremities (Table 2). Although patient’s clinical neurologic examination remained within normal limits, NCS abnormalities at her sensory peroneal nerve remained (Table 3).
Two years post her T1D diagnosis the girl complained again of paraesthesia in her upper and lower extremities which was considered a relapse of DPN attributed to a deterioration of her metabolic control (HbA1c 8.6%) possibly due to binge eating episodes despite her normal growth evolution (height: 75-90th percentile, weight: 50th percentile, BMI: 25-50th percentile). Apart from the residual impairment in the sensory fibers of the peroneal nerve, new NCS revealed additional dysfunction at sural nerve (Table 3). Although her symptoms gradually disappeared, her VST values remained pathologic (same values as 3 months post diagnosis, Table 2). The girl had no further neuropathic symptoms or chronic diabetic complications since.
Table 1: Clinical characteristics of patient A: a) ED with acute weight loss and APDN, b) 3 months later and c) 2 years later.
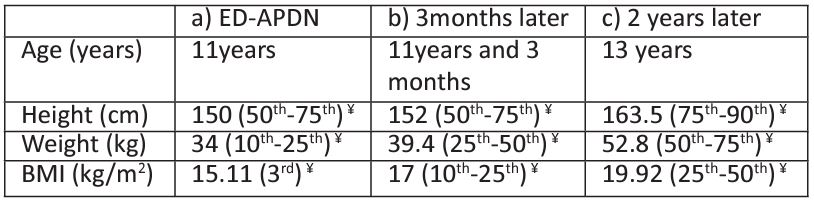
¥% percentiles according to published national data, ED: Eating Disorder, APDN: Acute Painful Diabetic Neuropathy.
Table 2: Vibration sensation thresholds (VST) in the upper and lower limbs of the patient: a) ED with acute weight loss and APDN, b) 3 months later and c) 2 years later.
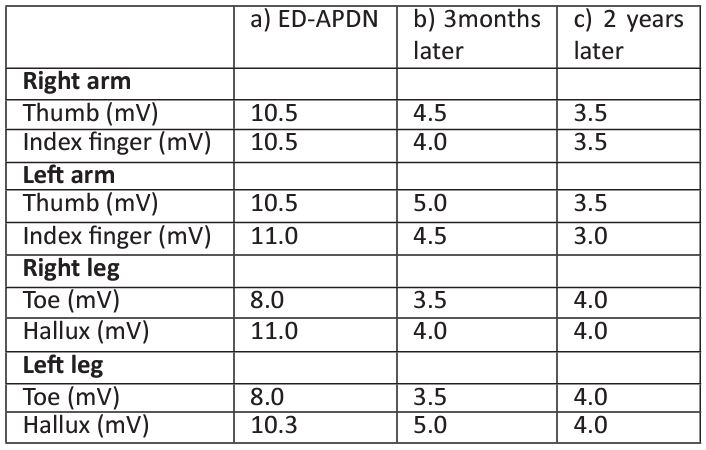
Values are the mean of three measurements in each site, ED: Eating Disorder, APDN: Acute Painful Diabetic Neuropathy.
Table 3: Nerve conduction studies in the peroneal (sensory and motor fibers), sural and median (sensory and motor fibers) nerves: a) ED with acute weight loss and DPN, b) 3 months later and c) 2 years later.
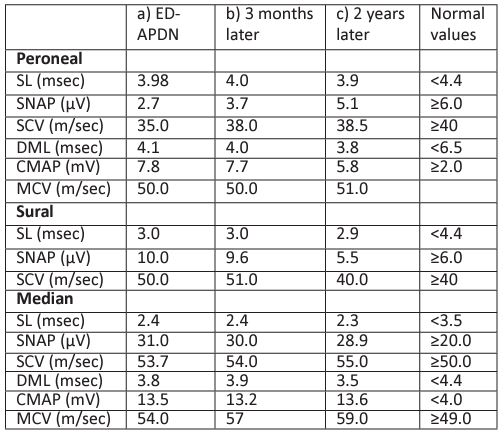
SL: sensory latency, SNAP: sensory nerve action potential (sensory amplitude), SCV: sensory conduction velocity, DML: distal motor latency, CMAP: compound muscle action potential (motor amplitude), MCV: motor conduction velocity, ED: Eating Disorder, APDN: Acute Painful Diabetic Neuropathy. Table 4: Clinical characteristics of patient B: a) T1D diagnosis with acute weight loss b) Microalbuminuria presence c) APDN diagnosis 3 years later d) 4years 5 months post T1D diagnosis. BMI: Body Mass Index, T1D:Type 1 Diabetes, APDN: Acute Painful Diabetic Neuropathy. Table 5: HbA1c% of patient B a) Microalbuminuria presence b) DPN diagnosis 3 years later c) 4years 5 months post T1D diagnosis. HbA1c%: Glycosylated Hemoglobulin A1c% ,T1D: Type 1 Diabetes, APDN: Acute Painful Diabetic Neuropathy.
90th percentile, weight: 50th percentile, BMI: 25-50th percentile). Apart from the residual impairment in the sensory fibers of the peroneal nerve, new NCS revealed additional dysfunction at sural nerve (Table 3). Although her symptoms gradually disappeared, her VST values remained pathologic (same values as 3 months post diagnosis, Table 2). The girl had no further neuropathic symptoms or chronic diabetic complications since.
CASE B
A 13-year-old boy was admitted to our hospital for a mild DKA episode accompanied by weight loss (-6 kg in two months,
Table 4: Clinical characteristics of patient B: a) T1D diagnosis with acute weight loss b) Microalbuminuria presence c) APDN diagnosis 3 years later d) 4years 5 months post T1D diagnosis.
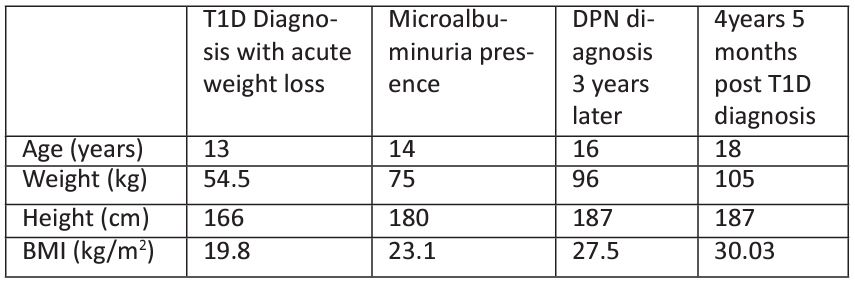
BMI: Body Mass Index, T1D:Type 1 Diabetes, APDN: Acute Painful Diabetic Neuropathy.
Table 5: HbA1c% of patient B a) Microalbuminuria presence b) DPN diagnosis 3 years later c) 4years 5 months post T1D diagnosis.
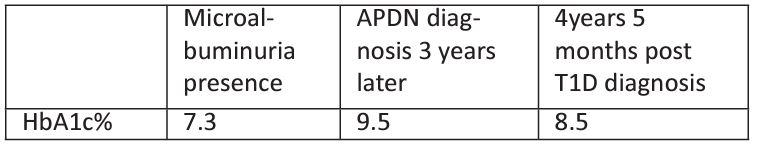
HbA1c%: Glycosylated Hemoglobulin A1c% ,T1D: Type 1 Diabetes, APDN: Acute Painful Diabetic Neuropathy.
weight: 54.5 kg). His capillary glucose was 407mg/dl and ABGs were as: pH 7.32, HCO3 24 mmol/L, ABE -2 mmol/L. Paediatric DKA management protocol was followed, and the patient responded excellent to treatment. He was discharged 7 days after with a T1D diagnosis and management plan. The boy was previously healthy, born at term with normal vaginal delivery. Although neonatal macrosomia (birth weight 4.8 kg) was confirmed, there was no history of maternal gestational Diabetes.
After the initiation of basal-bolus insulin regime, the boy regained weight (63.7 kg, 75th percentile for his weight) although he reported frequent recurrent hypoglycaemic episodes (HbA1c 5%). At his first year follow up appointment the patient excibited an adequate glycaemic control (HbA1c 7.3%,) had gained 20.5 kg (weight: 75kg, BMI 23.1 kg/m2) since diagnosis and developed microalbuminuria (45 mg/24hrs, normal value <30). Despite the optimization of his glycaemic control at year-two follow up appointment (HbA1c 5.9%), he had gained another 15kg (weight 90kg, BMI 27,17 kg/m2) and microalbuminuria persisted (44.0 mg/24hrs, normal value <30). Due to persisting microalbuminuria and to the patient’s best interest, an insulin pump-regime was offered by the diabetes medical team.
Three years after his T1D diagnosis and despite the initiation of insulin-pump the boy developed APDN. The patient’s glycaemic profile had deteriorated (HbA1c%: 9.5) and now complained of a 60day history of paraesthesia and pain at his lower and upper extremities mainly at night hours (Table 4). NCS revealed temporal dispersion of potentials as an indication of partial demyelination, despite normal conduction velocities. Although insulin pump regime was introduced early after T1D diagnosis, the boy developed painful DPN symptoms most likely as a result of combined poor glycaemic control and extreme weight gain possibly due to polyphagia episodes. Even though a Diabetes education refreshment course was offered, and a tighter medical supervision was applied by our multidisciplinary team his glycaemic control remained poor thereafter (Table 4). Four years and 5 months post T1D diagnosis, his HbA1c% remained high (8.5%) while he gained a significant amount of weight (weight 105kg, BMI 30.03 kg/m2, indicative of obesity). DPN symptoms remained unchanged thereafter, possibly due to extreme weight gain and persistent poor glycaemic control (Table 5).
Discussion
We hereby present 2 cases of adolescents with short T1D duration (9.8 months and 2 years respectively) and ED who developed APDN. Both patients had many risk factors for APDN that may have attributed to the early appearance of the disease such as early poor glycaemic control, rapid and extreme weight changes and poor compliance with insulin therapy. Based on international bibliography APDN cases are rare and reports of children or adults with recent onset diabetes and APDN are scarce [18]. Furthermore, cases of newly diagnosed T1D teenagers with early development of APDN and ED are even more rarely described in the literature.
APDN is a small-fiber polyneuropathy occurring within 8 weeks after rapid restoration of euglycemia, evident by a significant reduction in HbA1c (i.e a decrease in HbA1c ≥2% points over 3months) [18].
Patients with T1D are strongly advised to follow a strict diet and maintain normal weight in order to achieve normoglycemia which usually attributes to low self-esteem and increased levels of anxiety among young and especially female T1D patients [19, 20]. In addition, the clinical presentation of T1D precedes a glycosuria-related acute weight loss which is often perceived as desirable especially by female adolescent T1D patients [21]. Although, the initiation of insulin therapy is associated with weight gain and in combination with a dietary restraint predispose young T1D females to develop ED, Manucci et al reported significantly higher prevalence of Bulimia Nervosa among T1D female adolescents and adults (1.73%), when compared to non-diabetic population (0.69%) [22]. ED behavior in turn is often associated with poor glycemic control, higher levels of mean HbA1c and increased risk of microvascular complications in young T1D females [22]. Insulin omission as a measure of weight reduction has been reported in 27% of the female T1D participants in the study of Colton et.al [14]. There are several studies on disordered eating habits and misuse of insulin as a measure to control body weight among T1D adolescents [23]. DSM-V criteria categorise insulin omission as a well-recognised purging behaviour and the commoner weight reduction method following dieting, a behaviour similar to our female patient’s. As discussed above with our female patient, ED and acute diabetic complications, such as episodes of hypoglycaemia and Diabetic Ketoacidosis (DKA) are strongly related. DKA episodes may in turn eventually attribute to APDN presentation due to concomitant endothelial damage, hemodynamic and metabolic changes.[24] APDN has been furthermore associated with the presence of ED and weight changes.[25] Both our patients due to ED experienced extreme weight changes, had rapid drop in their HbA1c and poor glycemic control that precipitated the first APDN presentation.
Unfortunately, emotional distress is a major factor for the development of ED, affecting about 20–40% of T1D patients [26]. Anxiety symptoms alone have been related to higher levels of HbA1c, poor glycaemic control, depression, fear of hypoglycaemia, and poor glycose self-monitoring [19, 20]. Clinicians could refer these patients for Mindfulness-Based Cognitive Therapy (MBCT) or provide a simple eating habit questionnaire in order to detect the tendency to develop ED in the future.
Children and adolescents with long-term poor glycaemic control present early in their adolescent years with diabetic complications [27]. Our male patient experienced extreme weight gain and had poor metabolic control due to polyphagia in parallel with the development of microvascular long-term complications (microalbuminuria), in addition to the development of APDN.
According to Steel et.al , there were cases of young females with ED and poor glycaemic control who had an early onset of APDN, associated with pain during the peak of weight reduction and remission of pain with NCS improvement as weight was regained [28]. This finding is consistent with our female patient who indeed experienced partial remission of her DPN symptoms after gaining back weight. The improvement in NCS in these patients is indicating that the optimization of glycemic control might restore the neural impairment. In that view “Metabolic Memory” (a term describing the long-term effects of tight glycemic control on the prevention of diabetic neuropathy) may explain the partial improvement of the amplitude and conduction velocity in the sensory peroneal nerve 3 months after the deranged glycemic control in our female patient (table 2) [29]. The above may explain why the DPN symptoms were partially resolved following a short-period of near-normoglycemia (table 3) and why they progressively deteriorated again resulting in NCS impairment of the sural nerve. APDN has also been reported to occur after a significant drop in HbA1c following the commencement of insulin therapy or shortly after a major HbA1c drop at any stage of the disease. Furthermore in adults, acute improvement of glycemic control has been associated with the deterioration of diabetic retinopathy.[30]In this context, it may be worthwhile for physicians to embrace a slower and more gradual reduction in HbA1c when aiming for euglycemia, especially in poorly controlled patients with diabetes in order to prevent the occurrence of APDN or retinopathy .
In conclusion, APDN is a rare DM complication in youth, which may develop at any time during the course of the disease, even soon after diagnosis. It may appear after extreme weight changes usually in the context of ED, leading to chronic complications. Such was the case of our two T1D adolescents, in whom rapid deterioration of glycemic control in association with ED was a major risk factor for the development of APDN. Remission of symptoms occurred in both patients following restoration of optimal diabetic control and weight normalization. However, DN symptoms reoccurred, following future glycemic deterioration. Both of our patients with ED and glycemic control deterioration presented acute diabetic complications, including APDN, therefore requiring early management by expert clinicians and careful follow-up. Achieving an optimal metabolic control and maintaining a healthy living is the safest way to delay or even reverse DN at initial stages. As such, clinicians should carefully monitor weight changes among adolescents with T1D in order to early identify ED behaviour and stress symptoms and maintain a healthy lifestyle and well-being. Moreover, clinicians should advise patients to avoid rapid HbA1c changes (either improvement or deterioration) in order to prevent the occurrence of APDN.
Conflict of interest: The authors declare no conflict of interest.
Authorship Contributions: Writing: Maria Kaza, Data Collection: Spyridon Karanasios, Irine-Ikbale Sakou, Maria Louraki, Dimitra Kallinikou, Maria Kaza, Nerve conduction studies: Marina Katsalouli, Analysis or Interpretation: Kyriaki Karavanaki, Maria Kaza, Literature Search: Kyriaki Karavanaki, Maria Kaza, Maria Louraki, Design: Kyriaki Karavanaki, Maria Kaza, Concept: Kyriaki Karavanaki, Maria Kaza, Review and Editing : Kyriaki Karavanaki, Maria Kaza, Supervision: Kyriaki Karavanaki.
Financial Disclosure: The authors declared that this study received no financial support.
Ethics: No ethical issues were raised due to the nature of the study
Case presentation
- Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ (2010) Epidemiology of Type 1 Diabetes. Endocrinol Metab Clin North Am 39:481–497. https://doi.org/10.1016/j.ecl.2010.05.011
- American Diabetes Association, American Academy of Neurology (1988) Report and Recommendations of the San Antonio Conference on Diabetic Neuropathy. Diabetes Care 11:592–597. https://doi.org/10.2337/diacare.11.7.592
- Tesfaye S, Selvarajah D (2012) Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy: Advances in Epidemiology, Pathogenesis and Management of DPN. Diabetes Metab Res Rev 28:8–14. https://doi.org/10.1002/dmrr.2239
- Feldman EL, Callaghan BC, Pop-Busui R, et al (2019) Diabetic neuropathy. Nat Rev Dis Primer 5:41. https://doi.org/10.1038/s41572-019-0092-1
- Louraki M, Karayianni C, Kanaka-Gantenbein C, et al (2012) Peripheral neuropathy in children with type 1 diabetes. Diabetes Metab 38:281–289. https://doi.org/10.1016/j.diabet.2012.02.006
- Jaiswal M, Divers J, Dabelea D, et al (2017) Prevalence of and Risk Factors for Diabetic Peripheral Neuropathy in Youth With Type 1 and Type 2 Diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care 40:1226–1232. https://doi.org/10.2337/dc17-0179
- Ghaemi N, Hasanabadi H, Ashrafzadeh F, et al (2018) Peripheral Neuropathy in Children and Adolescents with Insulin-dependent Diabetes Mellitus. Iran J Child Neurol 12:83–90
- Verrotti A, Catino M, Di Ricco L, et al (1999) Prevention of microvascular complications in diabetic children and adolescents. Acta Paediatr Oslo Nor 1992 Suppl 88:35–38. https://doi.org/10.1111/j.1651-2227.1999.tb14338.x
- Singh R, Kishore L, Kaur N (2014) Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res 80:21–35. https://doi.org/10.1016/j.phrs.2013.12.005
- Wilmshurst JM, Ouvrier RA, Ryan MM (2019) Peripheral nerve disease secondary to systemic conditions in children. Ther Adv Neurol Disord 12:1756286419866367. https://doi.org/10.1177/1756286419866367
- Kobayashi M, Zochodne DW (2018) Diabetic neuropathy and the sensory neuron: New aspects of pathogenesis and their treatment implications. J Diabetes Investig 9:1239–1254. https://doi.org/10.1111/jdi.12833
- Porta-Etessam J, Garcia-Morales I, Martinez-Salio A, et al (2000) Gabapentin in acute painful diabetic neuropathy. Eur J Neurol 7:365–365. https://doi.org/10.1046/j.1468-1331.2000.00079.x
- Melmed S (2011) Williams textbook of endocrinology, 12. ed. Saunders Elsevier, Philadelphia, Pa
- Colton PA, Olmsted MP, Daneman D, et al (2015) Eating Disorders in Girls and Women With Type 1 Diabetes: A Longitudinal Study of Prevalence, Onset, Remission, and Recurrence. Diabetes Care 38:1212–1217. https://doi.org/10.2337/dc14-2646
- Pinhas-Hamiel O (2015) Eating disorders in adolescents with type 1 diabetes: Challenges in diagnosis and treatment. World J Diabetes 6:517. https://doi.org/10.4239/wjd.v6.i3.517
- Matsaniotis N, Karpathios T, Nikolaidou-Karpathiou P. (2010) Epitome pediatrics. Litsas publications
- Preston DC, Shapiro BE (2005) Electromyography and neuromuscular disorders: clinical-electrophysiologic correlations, 2. ed. Elsevier, Butterworth-Heinemann, Philadelphia
- Dayal D (2016) Acute Painful Neuropathy in a Girl with Type 1 Diabetes: Long Term Follow-Up. J Clin Diagn Res. https://doi.org/10.7860/JCDR/2016/19765.7773
- Buchberger B, Huppertz H, Krabbe L, et al (2016) Symptoms of depression and anxiety in youth with type 1 diabetes: A systematic review and meta-analysis. Psychoneuroendocrinology 70:70–84. https://doi.org/10.1016/j.psyneuen.2016.04.019
- Rechenberg K, Whittemore R, Grey M (2017) Anxiety in Youth With Type 1 Diabetes. J Pediatr Nurs 32:64–71. https://doi.org/10.1016/j.pedn.2016.08.007
- Verrotti A, Catino M, De Luca FA, et al (1999) Eating disorders in adolescents with type 1 diabetes mellitus. Acta Diabetol 36:21–25. https://doi.org/10.1007/s005920050140
- Mannucci E, Rotella F, Ricca V, et al (2005) Eating disorders in patients with Type 1 diabetes: A meta-analysis. J Endocrinol Invest 28:417–419. https://doi.org/10.1007/BF03347221
- Peveler RC, Fairburn CG, Boller I, Dunger D (1992) Eating disorders in adolescents with IDDM: A controlled study. Diabetes Care 15:1356–1360. https://doi.org/10.2337/diacare.15.10.1356
- Baszyńska-Wilk M, Wysocka-Mincewicz M, Świercz A, et al (2018) Peripheral Neuropathy as a Complication of Diabetic Ketoacidosis in a Child with Newly Diagnosed Diabetes Type 1: A Case Report. J Clin Res Pediatr Endocrinol 10:289–293. https://doi.org/10.4274/jcrpe.5374
- Tran C, Philippe J, Ochsner F, et al (2015) Acute painful diabetic neuropathy: an uncommon, remittent type of acute distal small fibre neuropathy. Swiss Med Wkly. https://doi.org/10.4414/smw.2015.14131
- van Son J, Nyklicek I, Pop VJ, et al (2013) The Effects of a Mindfulness-Based Intervention on Emotional Distress, Quality of Life, and HbA1c in Outpatients With Diabetes (DiaMind): A randomized controlled trial. Diabetes Care 36:823–830. https://doi.org/10.2337/dc12-1477
- Brink SJ (2001) Complications of pediatric and adolescent type 1 diabetes mellitus. Curr Diab Rep 1:47–55. https://doi.org/10.1007/s11892-001-0010-1
- Steel JM, Young RJ, Lloyd GG, Clarke BF (1987) Clinically apparent eating disorders in young diabetic women: associations with painful neuropathy and other complications. BMJ 294:859–862. https://doi.org/10.1136/bmj.294.6576.859
- Albers JW, Herman WH, Pop-Busui R, et al (2010) Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care 33:1090–1096. https://doi.org/10.2337/dc09-1941
- Chantelau E, Frystyk J (2005) Progression of diabetic retinopathy during improved metabolic control may be treated with reduced insulin dosage and/or somatostatin analogue administration – a case report. Growth Horm IGF Res 15:130–135. https://doi.org/10.1016/j.ghir.2004.12.005

