Research article - Volume 3 - Issue 2
Is Scaffolding Iliac Bone Graft Within Titanium Mesh Successful in Reconstructing Unilateral Large-Sized Mandibular Contour Defects?
Mohammed Qasem A1; Jiansuo H2,3; Yingyou H1; Wang Y1; Jihua Li4*
1Resident, State Key Laboratory of Oral Diseases and National Clinical Research Center for Oral Diseases, Center of Orthognathic and TMJ Surgery, West China Hospital of Stomatology, Sichuan University, Chengdu, P.R. China.
2Fellow surgeon, State Key Laboratory of Oral Diseases & National Clinical Research Center for Oral Diseases & Dept. of Orthognathic & TMJ Surgery, West China Hospital of Stomatology, Sichuan University, Chengdu, China.
3Attending surgeon, Department of Oral and Maxillofacial Surgery, Stomatology Medical Center, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China.
4Professor, State Key Laboratory of Oral Diseases and National Clinical Research Center for Oral Diseases, Center of Orthognathic and TMJ Surgery, West China Hospital of Stomatology, Sichuan University, Chengdu, P.R. China.
Received Date : Mar 06, 2023
Accepted Date : April 03, 2023
Published Date: April 06, 2023
Copyright:© Jihua Li 2023
*Corresponding Author : Jihua Li , Professor, State Key Laboratory of Oral Diseases and National Clinical Research Center for Oral Diseases, Center of Orthognathic and TMJ Surgery, West China Hospital of Stomatology, Sichuan University, Chengdu, P.R. China.
Email: leejimwa6698@sohu.com
DOI: Doi.org/10.55920/2771-019X/1413
Abstract
Objectives: The reconstruction of unilateral large-sized mandibular contour defects with no loss of continuity and good occlusion has been a challenge. The purpose of the current study was to evaluate the effect of using patient-specific titanium mesh scaffolding avascular pure iliac bone graft in reconstructing these defects.
Materials and Methods: From December 2017 to December 2020, thirteen patients with unilateral large-sized mandibular contour defects treated in Orthognathic and TMJ Surgery Center of West China Stomatology Hospital were enrolled in this retrospective study. Patient-specific titanium meshes and mandible models were designed and manufactured by computeraided design/computer-aided manufacturing based on reverse engineering after mirroring the unaffected mandibular side. Linear and angular measurements comparing preoperative and postoperative mandibular CT scans were used to analyze the symmetries of mandibular contour postoperatively.
Results: The measurements showed satisfactory 3D symmetries of mandibular contour with significant improvement of the facial symmetry as a whole compared to the preoperative status of all participating patients.
Conclusion: Titanium mesh scaffolding iliac crest bone graft is a successful and feasible combination for reconstructing unilateral large-sized mandibular contour defects.
Clinical Relevance: This approach can be added to surgeons’ armamentarium improving the reconstruction outcomes that will improve patients’ quality of life.
Key words : CAD/CAM; Mandibular defects; Patient-specific titanium mesh; Lliac crest bone graft; Reverse engineering.
Introduction
The appearance of the lower third of the face is mainly determined by mandibular bony contour, so patients with unilateral mandibular contour defects due to congenital hypoplasia, trauma, surgical resection, tumor, or any other etiology would suffer significant facial asymmetry [1]. This deformity is a significant clinical challenge for surgeons and patients [1]. The surgeons’ challenges include choosing the surgical method and materials to be used, which are associated with many demerits that will lead to patients’ challenges [2-5]. The benefits of titanium (Ti) and iliac crest bone graft in reconstructing craniomaxillofacial defects are well established in the literature [2-6]. However, no previous work used them simultaneously to reconstruct large-sized mandibular contour defects. As a trial to fill this gap, we combined both elements to gain the advantages of both, taking advantage of the recently refined reverse engineering concept and CAD/CAM technology to design and manufacture patient-specific titanium mesh scaffolding iliac crest bone graft to reconstruct such defects. We hypothesize that Ti mesh would expedite the iliac crest bone graft intake due to stable and intimate contact between the graft and underlying bone, ensuring restoration of mandibular symmetry and facial harmony. The study aimed to evaluate the effect of using reverse engineering generated patient-specific titanium mesh combined with iliac crest bone graft in correcting large-sized mandibular contour defects.
Materials, Patients, and Methods
Patients:
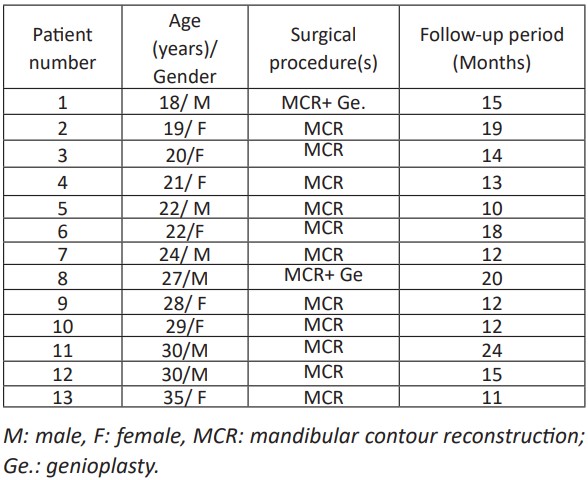
This retrospective study included thirteen patients (seven females and six males) who underwent surgical correction of unilateral mandibular bony contour deformity at Orthognathic and TMJ Surgery Center in Oral and Maxillofacial Surgery Department in West China Stomatology Hospital at Sichuan University (Chengdu, China) between December 2017 to December 2020 and all were 18 to 35 years of age (Table 1). Their medical and radiological records were retrieved from the registry system of the department. Inclusion criteria were 1. unilateral mandibular contour deformity that extended from mandibular angle to near the midline. 2. no history of facial bone trauma. 3. no preoperative malocclusion of facial asymmetry that could be corrected by conventional orthognathic surgery 4. availability of medical and radiological records. 5. patient permitted to use medical/radiological records for scientific publication. The exclusion criteria were 1. reconstruction with bone from any source other than the iliac crest. 2. history of any cosmetic or reconstructive surgery other than the technique described in the current study 3. incomplete records that do not provide all the data needed for the current study.
This study was approved by the institutional review board of the West China Hospital of Stomatology (WCHS-IRB-ST-2019-138). The study was conducted following the ethical principles of the Declaration of Helsinki (1964). All patients signed an informed consent agreement.
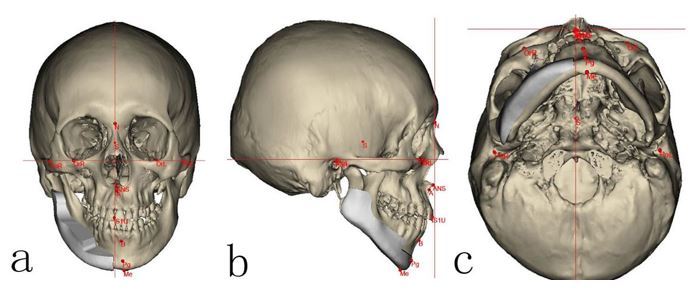
Figure 1: a. Frontal, b. sagittal c. axial plane 3D CT scans showing reverse engineering concept to design well-adapted titanium mesh over the affected mandibular side (right side) augmenting the deficient area to restore the symmetry based on mirroring the image of the healthy side of the mandible.
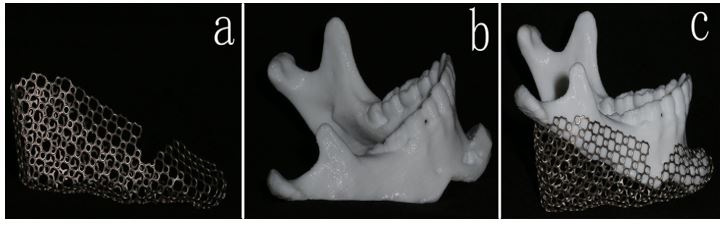
Figure 2: a.Printed out 3D Ti mesh. b. Printed out 3D mandibular model showing the simulated genioplasty. c. Titanium mesh adapted over the defective side of the mandible showing the space for the iliac bone graft.
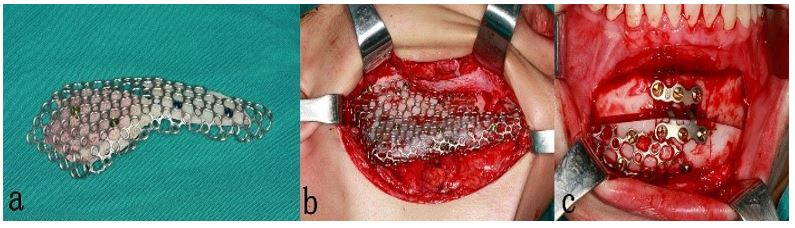
Figure 3: a.Titanium mesh with iliac crest bone graft blocks fixated with short titanium screws to the mesh. b. Titanium mesh scaffolding bone graft seated passively into the defective area intraoperatively. c. intraoperative photo after simultaneous genioplasty to correct the chin deviation (anterior extension of the mesh is fixated with short screws to enhance the stability).
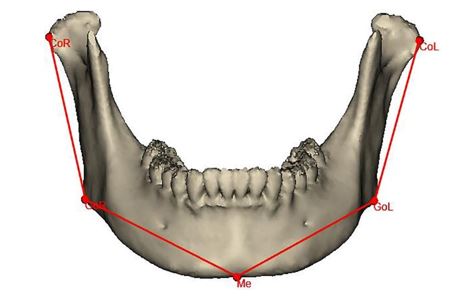
Figure 4: 3D CT scan showing the reference lines and angels to evaluate the success of the suggested surgical approach correcting unilateral mandibular contour defect to restore mandibular symmetry. Co-Go and Go- e lines (bilaterally) represent ramus length and mandibular body length, respectively. (CoR: Right Condylion, GoR: Right Gonion, Me: Menton, GoL: Left Gonion, CoL: Left Condylion).
Virtual Planning:
All patients’ facial bone spiral computed tomography (CT) scans done at two different times (T1: preoperative CT, T2: 12 months postoperative CT) at Medical Imaging Department in our hospital were collected as digital imaging and communications in medicine (DICOM) files. All CT images were captured by CT machine (Philips Brilliances 16, Best, The Netherlands) with the following parameters: 120 kV, 282 mm, and 26.3-seconds scan time. The collected DICOM data was then processed using MIMICS software v12.0 (Materialise, Belgium) to create a 3D model of the whole mandible to study the deformity's topography thoroughly. Then, the generated 3D model file was imported as stereolithography format (.stl) to be opened using 3 Matic™ software (Materialise, Belgium) to design Ti mesh based on the reverse engineering concept by which the mirror image of the contralateral side was cloned, ensuring good harmony and symmetry (Fig. 1). Finally, the Ti mesh was manufactured by CAM machine (PTY Medtech Co., Ltd. Shenzhen) and fitted over each patient’s mandibular printed 3D model that was used intraoperatively after standardized sterilization (Fig. 2).
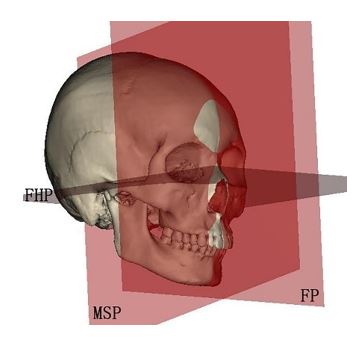
Figure 5: The three planes used to measure the angles formed with the lines shown in figure 4 to delineate the 3D position of the mandibular ramus and body postoperatively representing the aesthetic outcomes. (FHP: Frankfort horizontal plane, MSP: Mid-sagittal plane, FP: Frontal plane).
Surgical Techniques
The same senior surgeon corrected unilateral mandibular bony contour defects under general anesthesia using Ti mesh and pure autogenous bone graft harvested from the iliac crest. The patient’s face, neck, and iliac crest regions were prepared and draped in a routine sterile fashion. The oral cavity was isolated from the surgical site with sterile adhesive drapes. The mandibular defect site was approached through a cutaneous incision placed along the skin crease below the inferior border of the mandible. The soft tissues were widely undermined to prevent undue tension and ensure exposure of the whole contour defect site subperiosteally for better examination and proper adaptation and fixation of Ti mesh. Meanwhile, the pterygomasseteric sling and suprahyoid musculature were detached from the medial aspect of the lower border of the mandible only to the extent needed to adapt the titanium mesh appropriately. The marginal mandibular branch of the facial nerve and the mental nerve were protected during dissection, and both the facial artery and vein were ligated. Ti mesh fitness was checked, and any needed modifications were made to ensure stable mesh adaptation over the recipient bed. After having the iliac crest exposed in the usual manner, a prefabricated template was used to determine the shape and size of the corticocancellous bone block that needed to be harvested. Then, after good hemostasis, closure in layers with drain placement was done. The corticocancellous block was then trimmed and shaped to be adapted appropriately and fixated to the Ti mesh with short screws (Fig. 3 a.). Then, the whole unit was applied passively to the defect site, followed by fixation with multiple screws under copious irrigation with normal saline (Fig. 3.b). Meticulous hemostasis was accomplished at this time to minimize postoperative hematoma formation. Closure in layers after placement of negative pressure drain was followed. Two patients had genioplasty to correct the deviated chin (Fig. 3.c). Both drains were removed after 48 hours postoperatively.
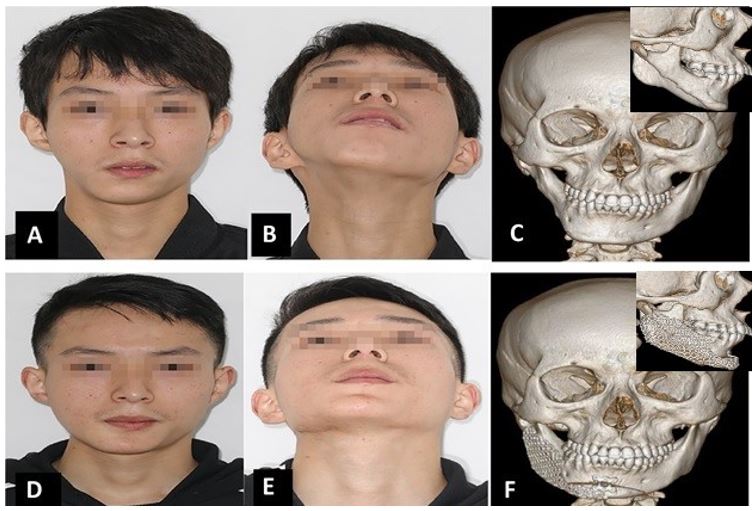
Figure 6: (a-b) Preoperative clinical photos showing the right mandibular contour deformity resulting in facial asymmetry with ipsilateral chin deviation that was depicted on 3D CT scan (c: frontal and lateral view). (d-e) Postoperative clinical photos show satisfactory symmetrical and aesthetic results after using titanium mesh with iliac crest bone graft and genioplasty to correct the chin deviation as shown on the postoperative 3D CT scan (f: frontal and lateral view showing augmented side)
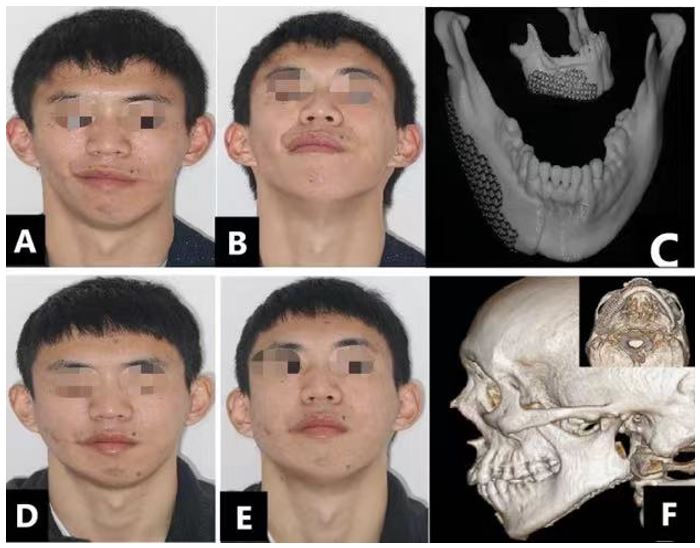
Figure 7: (a-b) Preoperative clinical photos showing the hypoplastic right side of the mandible due to contour deformity with resulting loss of mandibular angle definition. Titanium mesh trialed over printed out 3D mandible model (c: frontal and lateral view). (d-e) Postoperative clinical photos show satisfactory mandibular harmony and the facial aesthetic result after using titanium mesh with iliac crest bone graft as depicted on lateral and axial 3D CT scan (f).
Measured Data:
The measurement methods used in the current study were based on Nadia et al. study [7]. To verify the effectiveness of Ti mesh scaffolding iliac crest bone graft in restoring normal mandibular symmetry and harmony, we compared the reconstructed side dimensions with the normal side ones. So, Condylion (Co), Gonions (Go) bilaterally, and Menton (Me) in the midline were selected as landmarks to form the Co-Go reference line that represented the mandibular ramus length, and the Go-Me line to represent mandibular body length bilaterally (Fig. 4). Furthermore, to know the 3D positions of both mandibular ramus and body, we measured the angles formed between the mentioned lines and the mid-sagittal plane (MSP), Frankfort horizontal plane (FHP), and frontal plane (FP), as shown in figure 5. The linear and angular measurements were used to postoperatively measure the differences between the reconstructed and unaffected sides.
Statistical methods:
Statistical analysis was performed with an independent t-test using SPSS software version 25 (IBM, Chicago, IL, USA). Statistical significance was set at P- value < 0.05.
Table 1: The patients’ demographic, conducted operations, and length of the follow-up period details.
Results
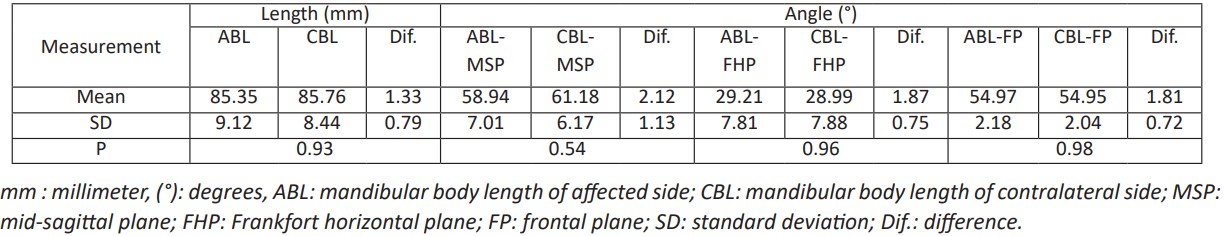
Seven female and six male patients with unilateral mandibular bony contour deformity underwent reconstruction with Ti mesh and iliac crest bone graft with a follow-up period of 10- 24 months (mean 15.6 months), and the average age was 24.8 years (range: 18-35 years) were included in this retrospective study. Subjective evaluation at one year postoperatively showed that the facial appearance of all patients improved dramatically (Fig. 6 d and e), and postoperative CT revealed that Ti mesh and integrated bone graft were in the planned position with satisfactory mandibular contour (Fig. 6 f). Out of the thirteen patients, only two patients needed genioplasty to adjust their deviated chins. There were no statistically significant differences in mandibular measurements (length of both Co-Go and Go-Me lines, MSP, FHP, and FP formed angels with mentioned lines) between the reconstructed and healthy sides (P>0.05) (Table 2 and Table 3). The patients' satisfaction was high, and their quality of life was substantially improved, as reported by the patients in the follow-up records. The interpretation of follow-up CT scans revealed active osteogenesis and a significant intake of the bone graft within the stable Ti mesh in intimate and stable contact with the recipient bed.
Representative Cases:
Patient 1: Right side mandibular bony contour deformity corrected by Ti mesh with iliac crest bone graft and genioplasty:
An 18-year-old male patient presented with a right-sided mandibular contour defect. Clinical examination showed severe asymmetry of the facial lower third with an ipsilateral deviation of the chin and significant contour defect of the right side of the face (Fig.6. a and b). Intraoral examination revealed no maxillary canting, no malocclusion, or bite abnormalities. Radiological examination showed a short and narrow (buccolingual) right mandibular ramus and body, with marked chin deviation from the normal side (Fig. 6.c1-c2). Postoperatively, the titanium mesh combined with the iliac crest bone graft augmented these contour defects. At the same time, genioplasty placed the chin's midline into a more harmonious position. The patient stayed in bed for one week, and normal gait was restored after four weeks with an uneventful recovery. A satisfactory patient facial appearance was achieved, which significantly enhanced self-confidence and patients’ quality of life as reported by the patient (Fig. 6.d and e). Besides, postoperative CT scans showed that chin deviation and contour defects were properly corrected, restoring facial symmetry (Fig. 6 f1-f2).
Table 2: The linear and angular differences of mandibular ramus between the affected and contralateral side postoperatively.
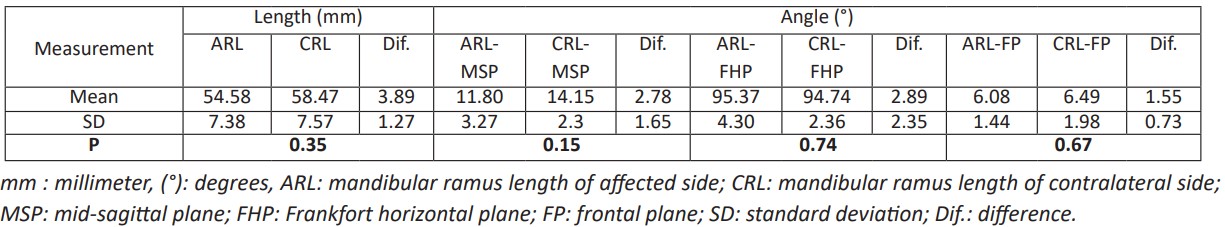
Table 3: The linear and angular differences of the mandibular body between the affected and contralateral side postoperatively.
Patient 2: Facial asymmetry due to mandibular contour deformity corrected by Ti mesh and iliac bone graft:
A 24-year-old male patient was referred to our hospital seeking correction of asymmetric mandibular contour deformity (Fig. 7.a and b). Radiological examination showed right-side mandibular body defects with a proper occlusal relationship. Individualized titanium mesh was designed and manufactured with the help of CAD/CAM based on reverse engineering (Fig. 7 c1-c2). Ti mesh scaffolding iliac crest bone graft was stabilized to achieve the optimum treatment plan goals. At seven months follow-up visit, the patient was satisfied with the surgical outcomes (Fig. 7.d and e). Furthermore, a follow-up CT scan showed no bone resorption and no changes in the mesh position with good augmentation and harmony with the other side (Fig. 7 f1-f2).
Discussion
The main objective of reconstructing unilateral mandibular contour defects is to restore the proper mandibular contour, achieving a stable long-term harmonious facial symmetry with minimal secondary procedures and donor site morbidities. Various modalities of reconstruction have been attempted, and each has its drawbacks [8].
Autogenous bone grafts are the golden standard for reconstructing facial skeletal defects. They have advantages over allogeneic implants, such as being immunologically compatible, structurally sound, free from risk of infection, and usually have an excellent fusion rate within the recipient site [9].
However, these grafts have unpredictable resorption, increased displacement rate, donor-site morbidity, difficulty in molding and securing into position, and prolonged surgical time [10, 11].
On the other hand, alloplastic materials are more readily available, lack donor-site morbidity, decrease surgical time and cost, and have relatively good postoperative tissue tolerance. Therefore, allogeneic implants have become an integral part of facial aesthetic surgery. Common alloplastic implant materials include silicone, Gore-Tex (expanded polytetrafluorethylene), and MedPor (polyethylene). However, these materials pose certain complications based on their surface contour (smooth vs. porous), flexibility, and reactivity with the surrounding tissue.
Smooth-surfaced implants (silicone) lack fibrous tissue ingrowth compared to porous implants (MedPor and Gore-Tex) [11-13]. The ingrowth of surrounding tissues helps stabilize the implant, and experimental data suggest that the presence of host defenses within the implant can decrease the risk of infection [13]. Thus, silicone implants are easily carved and removed, but their smooth surface predisposes them to migration and extrusion that jeopardize the aesthetic results [11, 13]. The natural body response to silicone implants is a chronic inflammatory process (peri-implantitis) leading to thick fibrous encapsulation, which increases the risk of late-onset infection compared to porous implants [12-15]. On the other hand, Gore-Tex and MedPor are more stable, lack capsule formation, have good biocompatibility, and have a lower risk of exposure [14-16]. While solid alloplastic implants can reconstruct small to medium-sized defects, they seem to fail in reconstructing significant defects [17]. The medium-sized pore Gore-Tex (10-30µm) and the hydrophobic nature make them less biocompatible with higher infection risk as the entry of macrophages will be reduced compared to MedPor (100-300µm) [18]. Bone resorption under solid implants has been reported to be more associated with silicone implants than Gore-Tex or MedPor. Gore-Tex implants are expensive and can harden when they contact blood, making them more palpable within subcutaneous tissue, as illustrated in Yang et al. study [19]. In our previous study, a computer-assisted individualized MedPor implant was reported to reconstruct mandibular contour defects with success [20]. However, MedPor showed no direct osseointegration and limited augmentation area size (< 8 cm) and was radiolucent on x-ray films and conventional CT scans making postoperative evaluation difficult [21].
These drawbacks primarily result from using biologically inactive and alloplastic materials to camouflage the skeletal defects rather than biologically active tissues regenerating the deficient skeleton. Therefore, a novel strategy is needed to provide potent pro-osteogenic signals within a scaffold of appropriate osteogenicity and structural quality material to minimize unsatisfactory results that lead to patients' morbidities like frequent medical consultations, multiple surgeries, and poor quality of life [1].
As a trial to approach this novel strategy, we combined titanium and iliac bone grafts to gain their advantages, restoring the native mandibular harmonious symmetry. Dale used titanium mesh to correct mandibular asymmetry in conjunction with orthognathic surgery and achieved sound therapeutic effects [22]. Each patient's mandibular bone topography is different from others; therefore, we used the reverse engineering concept and CAD/CAM techniques to design and fabricate the Ti mesh based on mirroring the normal side of the mandible. Furthermore, the well-adapted patient-specific Ti mesh has reduced graft resorption and enhanced the bone graft integration by preventing its displacement and keeping it in intimate contact with the recipient bed throughout the healing period [23, 24]. While most studies have focused on using Ti mesh to reconstruct mandibular discontinuity defects with iliac crest bone graft [25-29], the current study results demonstrate that the combination of Ti mesh with iliac crest bone graft has successfully restored the mandibular symmetry and harmony of clinically challenging cases of mandibular contour defects with no postoperative complications but pain, swelling, and little gait disturbance due to harvesting bone from the iliac crest, which improved within a short period. Analysis of postoperative CT scans showed no significant differences in mandibular ramus and body linear and angular measurements (P >0.05) between the reconstructed and normal sides. Furthermore, this technique can improve reconstruction outcomes and reduce the number of revisions, thus, reducing the psychological stress and pain of patients and reducing the surgical time and medical cost.
Although the present findings support this technique’s effectiveness, we know that this technique is not without limitations. One limitation is the unsatisfactory midline adjustment, which necessitates simultaneous genioplasty to correct the chin midline point. Also, the problem of soft tissue fullness cannot be entirely solved by this technique. However, as the soft tissue is a drape for the underlying hardware, if the deficient bone is adequately augmented as in the current process, the soft tissue will follow its new position and take the established contour.
We recommend further studies with larger sample sizes and extended follow-up periods to enhance the generalizability of the current results and to study the long-term effect of combining other surgical procedures with this technique to overcome these limitations.
Conclusion
Using patient-specific Ti mesh scaffolding iliac crest bone graft is a successful combination that provides optimal clinical outcomes for reconstructing unilateral large-sized mandibular contour defects.
Authorship confirmation statement
The authors confirm their contribution to the paper as follows:
all authors contributed equally to data curation, investigation, and resources. LJ, MQA, JH, HY, and YW authors contribute to conceptualization and visualization. MQA, JH authors did the methodology part. MQA and HY authors did the software analysis of the data. Formal analysis of the data was done by MQA and YW authors. Writing the original draft was done by MQA and JH authors. Reviewing and editing of the manuscript were done by LJ and MQA authors. Fund raising, project administration, supervision, and validation were the responsibility of LJ author (the corresponding author). All co-authors reviewed the results and approved the final version of the manuscript prior to submission.
Authors’ disclosure (Conflict of Interest) statement:
Conflict of Interest: The authors declare that they have no conflict of interest.
Consent to publish: The authors affirm that human research participants provided informed consent for publication of the images in Figure (6) and (7).
Acknowledgments: This study was supported by Key R & D Projects of Sichuan Provincial Department of Science and Technology (2020YFS0079).
References
- Gupta DM, Panetta NJ and Longaker MT (2009) The use of polymer scaffolds in skeletal tissue engineering applications. Journal of Craniofacial Surgery 20:860-861. https://doi: 10.1097/SCS.0b013e3181a9574b
- Arcas Pons A, Vendrell G, Cuesta F, Bermejo L and Piqué i Clusella N (2020) Mandibular angle augmentation using customized PEEK implants and guides generated with 3D Planning and Printing: case estudies. Annals of Case Reports, 2020, vol 14, p 511. https://doi.org/10.29011/2574-7754.100511
- Fu X, Qiao J, Girod S, Niu F, feng Liu J, Lee GK and Gui L (2017) Standardized protocol for virtual surgical plan and 3-dimensional surgical template–assisted single-stage mandible contour surgery. Annals of Plastic Surgery 79:236-242. https:// doi: 10.1097/SAP.0000000000001149
- Mommaerts MY (2016) Guidelines for patient-specific jawline definition with titanium implants in esthetic, deformity, and malformation surgery. Annals of maxillofacial surgery 6:287. https:// 10.4103/2231-0746.200325
- Woo JM, Baek S-H, Kim J-C and Choi J-Y (2018) Contour Restoration of Over-Resected Mandibular Angle and Lower Border by Reduction Mandibuloplasty Using Three-Dimensional Planning and Computer-Aided Design and Manufacturing Custom-Made Titanium Implants. Journal of Craniofacial Surgery 29:e340-e343. https://10.1097/SCS.0000000000004283
- Watzinger F, Luksch J, Millesi W, Schopper C, Neugebauer J, Moser D and Ewers R (2000) Guided bone regeneration with titanium membranes: a clinical study. British Journal of Oral and Maxillofacial Surgery 38:312-315. https://doi.org/10.1054/bjom.1999.0228
- Abou Kheir N and Kau CH (2014) Measuring mandibular asymmetry in Class I normal subjects using 3D novel coordinate system. Annals of Maxillofacial Surgery 4:34. https://10.4103/2231-0746.133073
- Sterodimas A, Huanquipaco JC, de Souza Filho S, Bornia FA and Pitanguy I (2009) Autologous fat transplantation for the treatment of Parry-Romberg syndrome. Journal of Plastic, Reconstructive & Aesthetic Surgery 62:e424-e426. https:// 10.1016/j.bjps.2008.04.045
- Yotsuyanagi T, Yamashita K, Urushidate S, Yokoi K and Sawada Y (2001) Reconstruction of large nasal defects with a combination of local flaps based on the aesthetic subunit principle. Plastic and reconstructive surgery 107:1358-1362. https://10.1097/00006534-200105000-00005
- Ridwan-Pramana A, Wolff J, Raziei A, Ashton-James CE and Forouzanfar T (2015) Porous polyethylene implants in facial reconstruction: outcome and complications. Journal of Cranio-Maxillofacial Surgery 43:1330-1334. https://doi.org/10.1016/j.jcms.2015.06.022
- Yaremchuk MJ (2003) Facial skeletal reconstruction using porous polyethylene implants. Plastic and reconstructive surgery 111:1818-1827. https://10.1097/01.prs.0000056866.80665.7a
- Rubin JP and Yaremchuk MJ (1997) Complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery: a comprehensive review of the literature. Plastic and Reconstructive Surgery 100:1336-1353. https://10.1097/00006534-199710000-00043
- Serna EM, Pliego ES, Ulldemolins NM and Morán AM (2008) Treatment of chin deformities. Acta Otorrinolaringologica (English Edition) 59:349-358.https://doi.org/10.1016/S2173-5735(08)70252-7
- Berghaus A and Stelter K (2006) Alloplastic materials in rhinoplasty. Current opinion in otolaryngology & head and neck surgery 14:270-277. https://10.1097/01.moo.0000233599.14671.4a
- Whitaker LA (1987) Aesthetic augmentation of the malar-midface structures. Plastic and reconstructive surgery 80:337-346. https://10.1097/00006534-198709000-00001
- Tantawi D, Eberlin S and Calvert J (2015) Midface implants: surgical and nonsurgical alternatives. Clinics in Plastic Surgery 42:123-127. https://doi.org/10.1016/j.cps.2014.09.005
- Couldwell WT, Chen TC, Weiss MH, Fukushima T and Dougherty W (1994) Cranioplasty with the Medpor porous polyethylene Flexblock implant. Journal of neurosurgery 81:483-486. https://doi.org/10.3171/jns.1994.81.3.0483
- de Moraes Ferreira ACR, Muñoz XMJP, Okamoto R, Pellizer EP and Garcia Jr IR (2016) Postoperative complications in craniomaxillofacial reconstruction with medpor. Journal of Craniofacial Surgery 27:425-428. https://doi.org/10.54527/jdir.2020.39.3.29
- Yang P, Yang Q, Liu T, Zeng J, Bi B, Zhou Y, Guo Y and Chen L (2015) A modified technique for expanded polytetrafluoroethylene shaping in chin augmentation: parallel groove carving technique. Journal of Craniofacial Surgery 26:e146-e148. https://10.1097/SCS.0000000000001382
- Gao H, Li X, Wang C, Ji P and Wang C (2019) Mechanobiologically optimization of a 3D titanium-mesh implant for mandibular large defect: A simulated study. Materials Science and Engineering: C 104:109934. https://doi.org/10.1016/j.msec.2019.109934
- Vendemia N, Chao J, Ivanidze J, Sanelli P and Spinelli HM (2011) A method for visualizing high-density porous polyethylene (medpor, porex) with computed tomographic scanning. Journal of Craniofacial Surgery 22:73-76. https://10.1097/SCS.0b013e3181f6f5fc
- Stringer D and Brown B (2009) Correction of mandibular asymmetry using angled titanium mesh. Journal of oral and maxillofacial surgery 67:1619-1627. https://doi.org/10.1016/j.joms.2008.12.068
- Buser D, Dula K, Hirt HP and Schenk RK (1996) Lateral ridge augmentation using autografts and barrier membranes: a clinical study with 40 partially edentulous patients. Journal of oral and maxillofacial surgery 54:420-432. https://doi.org/10.1016/S0278-2391(96)90113-5
- Donos N, Kostopoulos L and Karring T (2002) Alveolar ridge augmentation using a resorbable copolymer membrane and autogenous bone grafts: an experimental study in the rat. Clinical Oral Implants Research 13:203-213. https://doi.org/10.1034/j.1600-0501.2002.130211.x
- Zhou L-b, Shang H-t, He L-s, Bo B, Liu G-c, Liu Y-p and Zhao J-l (2010) Accurate reconstruction of discontinuous mandible using a reverse engineering/computer-aided design/rapid prototyping technique: a preliminary clinical study. Journal of Oral and Maxillofacial Surgery 68:2115-2121. https://doi.org/10.1016/j.joms.2009.09.033
- Modabber A, Gerressen M, Stiller MB, Noroozi N, Füglein A, Hölzle F, Riediger D and Ghassemi A (2012) Computer-assisted mandibular reconstruction with vascularized iliac crest bone graft. Aesthetic plastic surgery 36:653-659. https://doi.org/10.1007/s00266-012-9877-2
- Boyne P (1997) Reconstruction of the maxilla and mandible. Quintessence Inc, Hanover Park:37-52.
- Schopper C, Goriwoda W, Moser D, Spassova E, Watzinger F and Ewers R (2001) Long-term results after guided bone regeneration with resorbable and microporous titanium membranes. Oral and Maxillofacial Surgery Clinics of North America 13:449-458. https://doi.org/10.1016/S1042-3699(20)30130-8
- Von Arx T, Walkamm B and Hardt N (1998) Localized ridge augmentation using a micro titanium mesh: a report on 27 implants followed from 1 to 3 vears after functional loading. Clinical Oral Implants Research 9:123-130. https://doi.org/10.1034/j.1600-0501.1998.090208.x

