Review article - Volume 3 - Issue 3
Nuclear morphometry and stereological analysis of gastrocnemius and pectoralis major muscle tissue from MDX mice supplemented or not with creatine for 16 weeks
Giovanna Cavalcanti Banov1; Kadu Ferreira Gonçalves Teixeira1; Dante Guarnieri1; João Vitor Monteiro de Oliveira1; Mariana Shmayev1; Maria Carolina Delforno1; Giovanna Camarotto Patah1; Amilton Iatecola2; Marcelo Rodrigues da Cunha1,2; Victor Augusto Ramos Fernandes1,2*
1Faculty of Medicine of Jundiaí, Jundiaí, São Paulo, Brazil.
2Nossa Senhora do Patrocínio University Center, Itu, São Paulo, Brazil.
3University of Guarulhos, Guarulhos, São Paulo, Brazil.
Received Date : Mar 21, 2023
Accepted Date : April 24, 2023
Published Date: May 01, 2023
Copyright:© Victor Augusto Ramos Fernandes 2023
*Corresponding Author : Victor Augusto Ramos Fernandes, Faculty of Medicine of Jundiaí, Jundiaí, São Paulo, Brazil.
Email: victorfernandes@g.fmj.br
DOI: Doi.org/10.55920/2771-019X/1428
Abstract
Duchenne muscular dystrophy is a genetic disease that causes a reduction in the expression of dystrophin, thus, with the reduction of the levels of this protein, a progression of muscle atrophy, reduction of strength and physical impairments is identified, which culminates in death from inability to contract the respiratory muscles. Creatine is a dietary supplement that helps maintain strength and recent findings indicate a potential anti-inflammatory effect of this amine. The present study aimed to compare the progress of muscular dystrophy in terms of inflammation, presence of intramuscular glycogen and tissue fibrosis in the gastrocnemius and pectoralis major muscles of MDX mice. We selected 20 MDX mice that were supplemented or not with creatine at a dosage of 0.3 grams per kilogram of body weight, for 16 weeks. After that, the gastrocnemius and pectoralis major were extracted for morphological and morphometric analysis. The inflammatory progress derived from Duchenne muscular dystrophy affects animals, even when supplemented, however, in a less forceful and disabling way, enabling them to have less functional damage. Nevertheless, the supplemented animals have a greater stock of intramuscular glycogen in the evaluated gastrocnemius tissue, a factor that corroborates with the survival and better quality of movement in these animals, given the availability of energy for the contraction of this musculature. Furthermore, a lower frequency and extent of tissue fibrosis is observed in supplemented animals, aspects that enable longer survival and qualify creatine, even in an experimental condition, as an auxiliary supplement in the treatment of a severe dystrophic condition, acting mainly in the maintenance of strength and reduction of local tissue fibrosis.
Keywords: Creatine supplementation; Duchenne Muscular Dystrophy; Skeletal muscle; Dystrophin; Inbred Mice mdx.
Introduction
Duchenne muscular dystrophy is a recessive genetic disease that manifests itself in the individual's sex chromosome, compromising the DMD gene, responsible for encoding dystrophin, an important structural protein located in the subsarcolemmal area of striated muscle cells [1]. Genetic defects in the DMD gene can occur through deletions, duplications, or point mutations, and currently, there is no cure for this disease, with the use of corticosteroids being the most effective treatment [2]. However, the prolonged use of this class of drugs can causeharmful effects on the patient, such as bone fractures, lesions in the lining epithelium of the gastrointestinal tract, and opportunistic infections [3].
Thus, it is assumed that the continuous use of corticosteroids, even though they are the most efficient therapy currently available in the treatment of this disease, it is fundamental to associate them with dietary supplements and vitamins that can benefit the patient and provide tissue repair [4]. In this sense, monohydrated creatine has been extensively investigated as a dietary supplement with potential tissue repair effects [5-8]. Creatine is an amine synthesized exclusively in eukaryotic cells, from the amino acids arginine, methionine, and glycine, and its formation occurs from the catalytic activity of the enzymes glycine amidinotransferase and guanidinoacetate-methyltransferase, which are abundant in hepatic, renal and pancreatic tissues [9].
Historically, creatine has been used as a food supplement for ergogenic purposes, given its ability to resynthesize adenosine triphosphate given the use of this energetic molecule in cellular activities [10]. Associated with this effect, creatine transports hydrogen ions and, therefore, provides a buffering effect on the intracytoplasmic environment9. Nevertheless, there is evidence that creatine enables the increase in the mammalian target protein pathway of rapamycin (mTOR), causing an increase in protein synthesis activity [11]. Thus, the effects of creatine benefit cellular homeostasis, together with its potential regenerative effect, mainly associated with this increase in protein synthesis.
The muscle tissue of patients with Duchenne muscular dystrophy is extensively impaired, mainly due to reduced or nonexistent synthesis of the dystrophin protein, which causes the lack of stabilization of the sarcolemma with the extracellular matrix of the endomysium. Consequently, there is a progressive reduction of the cytoplasm, which leads to myocyte atrophy and the development of tissue fibrosis [12]. Thus, it is observed that the muscle tissues of the lower limbs differ in the extent of impairment when compared to the upper limb muscles of the same patient, a fact that leads to a more aggressive reduction in muscle mobility in one region than in another. Another [13].
Thus, identifying possibilities for complementary, low-cost therapies with a potential regenerating effect become important in the investigation of this disease. In this sense, the present study aims to identify the effects of creatine monohydrate supplementation regarding the nuclear and cytoplasmic morphometry of the muscle tissue of the pectoralis major compared to the muscle tissue of the gastrocnemius, in the main animal model of investigation of Duchenne muscular dystrophy, mice mdx.
Material and Method
Experimental draw
In the study, 20 MDX mice (dystrophic mice) were used. It is noteworthy that this number of animals referred to other studies and recommended the minimum number for statistical purposes, because of the ethical regulations for animal use. The animals were organized into two groups:
● Group I: Consisting of MDX mice that received creatine monohydrate supplementation for sixteen weeks (CrMDX).
● Group II: Consisting of MDX mice that did not receive creatine monohydrate supplementation during the trial period (CoMDX).
All animals were in the four-week age group and had body mass duly standardized for the start of the experimental protocol (503.3g on average). The animals received a solid diet and water ad libitum and were kept in collective cages (5 mice of the same species per cage) at a constant temperature of 23±20C, a cycle of 12 hours light/12 hours dark, with lights on from 06:00 h to 18:00 h. All animals were fed Labina® (a standard rat chow diet supplied by Purina, Brazil). The body weights (g) of the animals were checked at the beginning and the end of the experiment. The animals were sacrificed one day after the last creatine supplementation, following the recommendations standardized by the ethics committee for animal use. The samples needed for analysis of the variables in this study (gastrocnemius and pectoralis major muscle tissue) were collected after anesthetic induction and sacrifice.
Painless death induced in animals
The animals had painless death induced one day after the experimental protocol, through a high dosage (0.3 mg/100gr) of anesthetic via intraperitoneal solution composed of Xylazine, Ketamine, and Thiopental. Subsequently, pneumothorax was induced in the animal. After confirming the death of the animals, the diaphragms were extracted for morphometric and stereological analysis.
Ethical aspects
The present study was approved by the Animal Use Ethics Committee under process 19/2021. The number of animals used followed the international precepts of animal use, respecting their proper use without exceeding and causing unnecessary interventions.
Creatine Supplementation
Supplementation of Creatine Monohydrate, at a dosage of 0.3mg per kg of body mass for 16 weeks, was administered to the animals in group I. The dosages were based on other studies using supplementation in rodents6,7 and are equivalent to the dosage regimen used in humans to obtain ergogenic effects [13]. Supplementation was administered by gavage, as proposed by Souza14 using an oroesophageal probe (1 mm in diameter; 3 cm in length) adapted to a 3 ml syringe, with water as the infusion vehicle. The animals in group I received creatine supplementation on Mondays, Wednesdays, and Fridays in the morning, between 06:00 and 10:00. Group II animals were submitted to the same gavage process, however only water was instilled.
Light Microscopy and Stereology
After the period of experimentation and euthanasia of the animals, the muscular tissues of the gastrocnemius and pectoralis major were extracted and these were then fixed in Bouin (Saturated aqueous solution of picric acid - 75 ml, formaldehyde - 25 ml, glacial acetic acid - 5 ml ) for 12 hours for further processing and embedding in paraffin. The tissues were then washed in 70% alcohol and subjected to dehydration in an increasing series of alcohols (80% alco
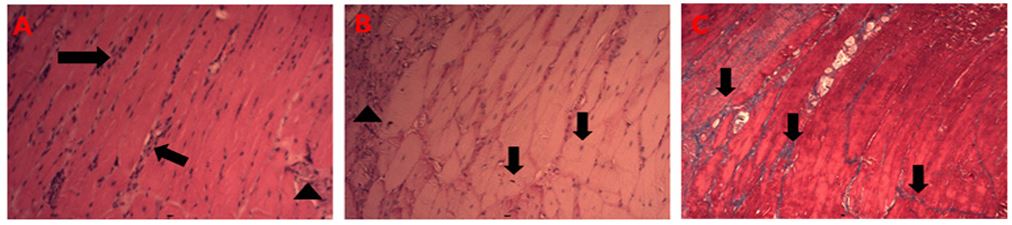
Figure 1– Group II: (A) Gastrocnemius muscle. Arrows indicate sites of inflammatory infiltration in the local endoderm. The arrow indicates fibrosis with the presence of mononuclear leukocytes. H/E, 20X objective. (B) Gastrocnemius muscle. The arrows indicate centralized nuclei, a fact that differs from the typical condition of this tissue. The arrow indicates fibrosis with the presence of mononuclear leukocytes. PAS, 20x objective. (C) Gastrocnemius muscle. Arrows indicate invasive tissue fibrosis. T. Masson, 10X objective.
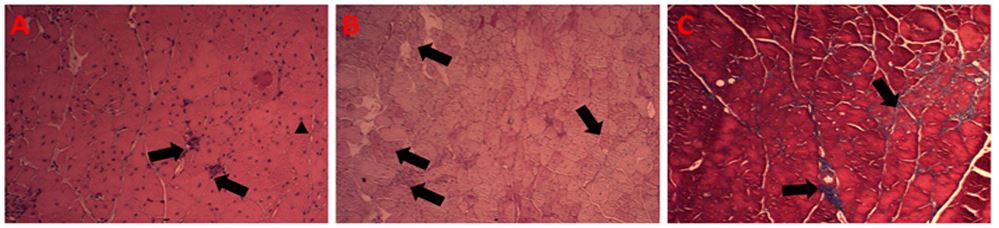
Figure 2 - Group I: (A) Gastrocnemius muscle. The arrow points to a myocyte with a centralized nucleus, a fact that differs from what is usual in this tissue. Arrows indicate the presence of mononuclear leukocytes describing an inflammatory infiltrate. 20x H/E objective. (B) Gastrocnemius muscle. The arrows indicate cells more stained with the selected method (PAS) in which a higher concentration of intramuscular glycogen is identified. PAS, 10X objective. (C) Gastrocnemius muscle. Arrows indicate tissue fibrosis. T. Masson, 20X objective.
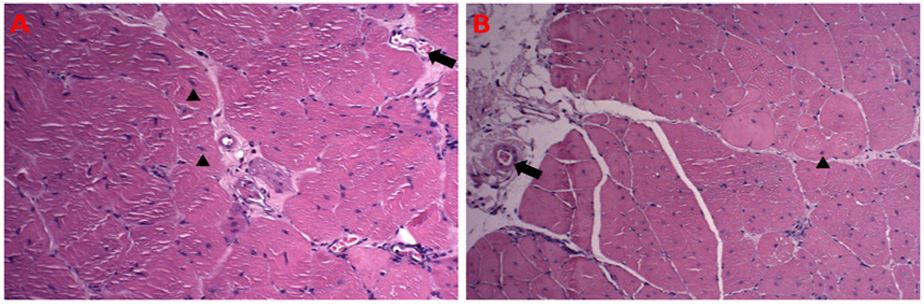
Figure 3: Comparison between groups II (A) and I (B) of the experimental protocol. Pectoralis major muscle. The arrows indicate vessels rich in red blood cells inside, showing possible inflammatory edema, given the mononuclear leukocytes surrounding the vessel. Arrows point to atypical nuclei of the evaluator tissue. H/E, 20X objective.
Table 1: Morphometric comparison between the experimental protocol groups, analysis of the Figure Figure 1: Gastrocnemius myocyte nucleus.
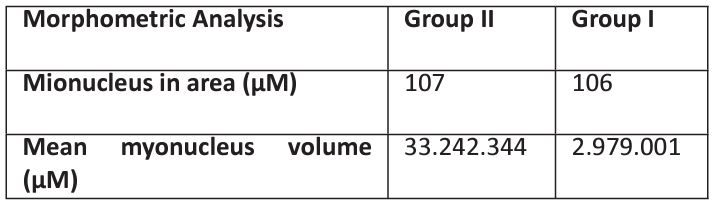
Morphometric analysis of the myocyte nuclei present in the gastrocnemius, comparison between groups. Comparison performed using the Analysis of Variance (ANOVA) statistical treatment as a parametric test, complemented with the Bonferroni Test involving the pairs of each group, assuming p<0.05.
Table 2: Morphometric comparison between the experimental protocol groups, analysis of pectoralis major myocyte nucleus.
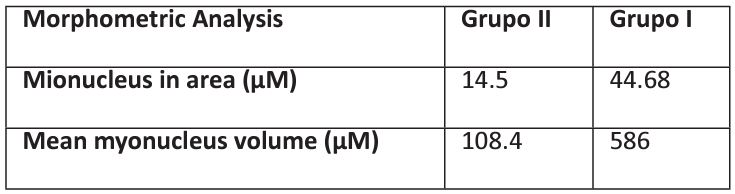
Morphometric analysis of the myocyte nuclei present in the pectoralis major, comparison between groups. Comparison performed using the Analysis of Variance (ANOVA) statistical treatment as a parametric test, complemented with the Bonferroni Test involving the pairs of each group, assuming p<0.05.
hol - 2 times, absolute alcohol - 3 times; 1 to 2 hours each) for inclusion. After that, the fragments were cleared in xylene for 1 to 2 hours until they became translucent. The fragments were then embedded in paraffin and plastic polymers (Paraplast Plus, Polysciences, Niles, IL) at 56°C for approximately 1 hour and then transferred to new paraffin at the same temperature. Tissues were carefully arranged at the bottom of plastic vats, to obtain cross-sectional histological sections. The blocks were trimmed to obtain flat surfaces and sectioned to five micrometers in thickness. Then the fragments were placed on albuminized slides and taken to the oven at 60°C. After preparing the sections, the slides were stained with hematoxylin/eosin (HE), Masson's Trichrome, and PAS for general morphological study. After obtaining the histological slides, they were analyzed and photographed, prioritizing the 10x and 40x objectives. All these cuts were used for morphometric and stereological analyzes of the extracted muscle tissues. Statistical analysis To obtain the average profiles between the groups, the Analysis of Variance (ANOVA) was used as a parametric test, complemented by the Bonferroni Test involving the pairs of each group, representing the non-parametric test. The entire study was performed with at least 5% significance.
Results
After applying the experimental protocol, euthanasia of the animals, staining protocols, and morphological analysis, the results were organized and are presented below.
Discussion
The pectoralis major muscle is the most extensive and superficial of the thorax, allowing adduction, medial rotation, and flexion of the humerus at the shoulder joint. It originates in two heads, one sternocostal and the other clavicular14.The first has fibers arising from the body of the sternum and the costal cartilages of the first seven ribs, whereas the clavicular head has fibers arising from the medial half of the bone. The fibers of both heads combine to insert into the intertubercular groove of the humerus [15]. The innervation of this important muscle is in charge of the medial and lateral pectoral nerves, both originating from the brachial plexus [14].
The gastrocnemius, in turn, is one of the large and strong superficial muscles of the leg. It assists in the execution of plantar flexion of the foot and flexion of the knee and has two heads, one medial and the other lateral, whose tendons originate respectively above the medial and lateral condyle on the posterior and distal face of the femur. These heads unite into a single tendon, which, in the lower part of the leg, converges its fibers to insert next to the soleus muscle in the tuberosity of the Achilles tendon, in the bone of the same name, at the level of the hindfoot. Its innervation is provided by the sciatic nerve, precisely by its tibial portion [14].
Although both muscles investigated in this study are skeletal striated muscles, they are affected in different ways by the progression of Duchenne muscular dystrophy. In this sense, the muscles of the pelvic girdle are initially affected, such as the levator ani muscles (puborectalis, pubococcygeus, and iliococcygeus), and of the lower limbs, such as the investigated gastrocnemius and others. It is identified that the affected musculature initially manifests a picture of pseudohypertrophy, which leads to overload of the knee extensor musculature and with this impairment of the gastrocnemius, which helps in gait biomechanics [1,2].
Later, in more advanced stages of muscular dystrophy, the involvement of the muscles of the upper limbs, such as the pectoralis major, is observed. This progress is followed by myocyte atrophy as well as a very severe acute inflammatory process, evidenced by the presence of mononuclear and polymorphonuclear leukocytes invading the muscle tissue [14,15]. Figure 1 presents the results observed in the group of MDX mice that were not supplemented with creatine monohydrate and had the gastrocnemius investigated. The findings describe, in chart A, an area rich in inflammatory infiltrates motivated by mononuclear leukocytes that are organized in rows, following the direction of the muscle fiber (in the longitudinal section shown). Table B of Figure 1 shows the PAS staining, which is applied to identify fibers rich in glycogen. The literature describes that the fibers with glycogen become darker, due to the interaction of the dye with the glucose polymers. As shown in chart B, there are few muscle fibers with this condition, predominating fibers in a more opaque situation, indicating a potential reduction of intramuscular glycogen in these fibers. These findings corroborate the literature that indicates a reduction in the strength and availability of effective contraction of the striated muscles in patients with muscular dystrophy. However, a new possibility is added that, in addition to the impairment of dystrophin that prevents adequate anchorage of the sarcoplasm to the local endomysium, there are also metabolic alterations associated with energy stores in these cells affected by muscular dystrophy. Chart C in Figure 1 indicates, using Masson's trichrome technique, extensive areas of fibrosis in the muscle tissue. These three components described are essentially present in the progression of muscular dystrophy and cause severe functional impairments in the patient [4,5].
Figure 2, in turn, shows the gastrocnemius of animals supplemented with creatine during the period of the experimental protocol. These findings were also organized into three histological techniques that allow identifying the presence of inflammatory infiltrates (chart A), intramuscular glycogen (chart B), and tissue fibrosis (chart C). As observed, it is possible to identify areas of inflammatory infiltration in the muscle tissue of supplemented animals, however, less extensive and frequent, when compared to animals in the non-supplemented group. Table A presents the situation and shows that the arrows show smaller areas of mononuclear leukocytes in the local tissue. Another important aspect observed is a smaller extent of tissue fibrosis, indicated by chart C, which demonstrates that there was less tissue repair after the chronic inflammation that sets in in this pathological condition. However, the finding that suggests the greater impact of these results is associated with chart B of figure 2. This chart indicates several myocytes stained in dark tones by the PAS technique, indicating a greater presence of local glycogen concentration [1,2,15].
It is known that creatine monohydrate is a naturally occurring amine and that it can be synthesized endogenously in the liver, kidneys, and pancreas. However, its supplementation substantially increases its intramuscular and other cell stocks, such as the nerve and seminiferous tubules present in the testicles. The increase in the intracytoplasmic supply of creatine facilitates the resynthesis of adenosine triphosphate (ATP), through the phosphocreatine pathway, a factor that makes more ATP available for cellular activities, without the need to activate glycolysis for short-term activities. In this way, the intramuscular glycogen stores remain high and the ATP levels as well, decisive factors in the maintenance of the individual's strength [4,10,15].
As described in the literature, figure 3 indicates that the pectoralis major muscle of both groups was quite similar, even though the muscle tissue of the supplemented group had larger nuclei and sarcoplasm, as shown in Table 2. , in the gastrocnemius, as shown in Table 1, are no longer distant between the groups, making it possible to identify an approximation of values, which do not differ statistically when comparing the groups of the experimental protocol [13,15].
Conclusion
The inflammatory progress derived from Duchenne muscular dystrophy affects animals, even when supplemented, however, in a less forceful and disabling way, enabling them to have less functional damage. Nevertheless, the supplemented animals have a greater stock of intramuscular glycogen in the evaluated gastrocnemius tissue, a factor that corroborates with the survival and better quality of movement in these animals, given the availability of energy for the contraction of this musculature. Furthermore, a lower frequency and extent of tissue fibrosis is observed in supplemented animals, aspects that enable longer survival and qualify creatine, even in an experimental condition, as an auxiliary supplement in the treatment of a severe dystrophic condition, acting mainly in the maintenance of strength and reduction of local tissue fibrosis.
Declaration of Conflict of Interests: The authors declare they have no conflicts of interest.
Financing: This research has not received any specific grants from agencies in the public, commercial, or for-profit sectors.
Ethical responsibilities: The authors declare that they carried out the studies in accordance with the national and international precepts of ethics in research with animals and with the support of the Ethics Committee for the Use of Animals, as stated in the manuscript.
References
- Falzarano MS, Scotton C, Passarelli C, Ferlini A. Duchenne Muscular Dystrophy: From Diagnosis to Therapy. Molecules. 2015; 20(10): 18168-84.
- Fernandes VAR, Delforno MC, Banov GC, et al. Renal, hepatic and muscle effects of creatine Supplementation in an older adults experimental model. Clinical Nutrition ESPEN. 2022; 48(01). Fairman CM, Kendall KL, Newton RU, Hart NH, Taaffe DR, Chee R, et al. Examining the effects of creatine supplementation in augmenting adaptations to resistance training in patients with prostate cancer undergoing androgen deprivation therapy: a randomised, double-blind, placebo-controlled trial. BMJ Open. 2019; 9(9): e030080.
- Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013; 173(5): 355-361.
- Kazak L, Cohen P. Creatine metabolism: energy homeostasis, immunity and cancer biology. Nat Rev Endocrinol. 2020; 16(8): 421-436.
- Trefts E, Gannon M, Wasserman DH. The liver. Current Biology. 2017; 27(21): R1147-51.
- Brunt EM, Gouw ASH, Hubscher SG, Tiniakos DG, Bedossa P, Burt AD, et al. Pathology of the liver sinusoids. Histopathology. 2014; 64(7): 907-20.
- Yoshizumi WM, Tsourounis C. Effects of creatine supplementation on renal function. J Herb Pharmacother. 2004; 4(1): 1-7.
- Fernandes VAR, Belozo FL, Conte M, Caldeira EJ. Estereologia e morfometria do tecido muscular-esquelético de animais submetidos a um programa de treinamento de força e suplementação de creatina durante 9 semanas. Revista Brasileira de Fisiologia do Exercício. 2019; 18(4).
- Sumien N, Shetty RA, Gonzales EB. Creatine, Creatine Kinase, and Aging. Subcell Biochem. 2018; 90: 145-168.
- Dawley C. Myalgias and Myopathies: Rhabdomyolysis. FP Essent. 2016; 440: 28-36.
- Comim CM, Ventura L, Freiberger V, Dias P, Bragagnolo D, Dutra ML, et al. Neurocognitive Impairment in mdx Mice. Mol Neurobiol. 2019; 56(11): 7608-7616.
- Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015; 4: 180-3.
- Ursini F, Maiorino M, Forman HJ. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016; 8: 205-15.
- Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, et al. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J Int Soc Sports Nutr. 2017; 14: 18.

