Case report - Volume 3 - Issue 3
Utilization of ActiGraft, an Autologous Whole Blood Clot, for Treatment of Complex Wounds Linked to Comorbidities
Emre Ozker*
Acıbadem Altunizade Hastanesi Hospital, İstanbul, Turkey.
Received Date : Mar 24, 2023
Accepted Date : April 25, 2023
Published Date: May 02, 2023
Copyright:© Emre Ozker 2023
*Corresponding Author : Emre Ozker, Acıbadem Altunizade Hastanesi Hospital, İstanbul, Turkey.
Email: scohen@reddressmedical.com
DOI: Doi.org/10.55920/2771-019X/1429
Abstract
Introduction: Delayed wound healing can be attributed to the patient’s comorbidities, having a negative effect on the body’s normal wound healing process. While the normal healing process goes through four phases, the chronic wound is not progressing from the inflammatory phase, with constant destruction of the extracellular matrix (ECM), not allowing a scaffold to cover the wound and protect it from bacteria bioburden.
ActiGraft is an autologous whole blood clot (AWBC) therapy, created from the patient’s peripheral blood in an outpatient setting. The AWBC contains all the factors required for efficient wound repair and has been shown to reduce wound size, and expedite wound closure. The AWBC protects the wound from infection, provides a temporary ECM, and modulates the immune response.
Objectives: In this case series, we show the efficacy of ActiGraft in treating complicated chronic wounds associated with various comorbidities.
Materials and Methods: 4 patients with multiple comorbidities signed an informed consent. 15 mL of blood was drawn and was activated ex vivo to create a whole blood clot. The clot was attached to the wound bed using steri-strips and was covered with a non-adherent pad and a secondary dressing foam. Re-application was conducted weekly.
Results: ActiGraft treatment was applied on chronic wounds with a background of severe comorbidities having a major effect on the wound healing process: deep vein thrombosis post-cancer-related surgical wound, peripheral arterial disease, and Charcot foot. ActiGraft treatment resulted in a reduction in wound size and wound progression in a timely manner.
Discussion: ActiGraft treatment was found to initiate and enhance the delayed healing process of complex and chronic wounds in patients suffering from comorbidities. ActiGraft creates a protective scaffold, restoring the homeostasis in the surrounding area of the wound, resulting in the initiation of the wound healing cascade in stagnant wounds.
Introduction
Chronic, non-healing wounds are defined as wounds that have not progressed through the normal healing process and are open for over a month, affecting over 2% of the worldwide population [1,2], with prevalence increasing with age [3,4]. Data from 2014 calculated that Medicare expenditure for all wounds ranges from $28.1 to $96.8 billion, including costs for infection management [5]. The EU financial burden was estimated at €4-6 billion in 2009 [6]. Millions of people in the US and Europe are affected by chronic wounds each year, such as diabetic foot ulcers, venous leg ulcers, arterial insufficiency, and pressure ulcers. Among these, surgical wounds and diabetic ulcers are the most expensive to treat [5,7]. Most chronic wounds are associated with comorbidities, including diabetes, venous insufficiency, peripheral arterial disease, cardiopulmonary and oxygen transport conditions, immune deficiencies, and dementia [4].
Comorbidities can delay wound healing by complicating and delaying diagnosis and negatively affecting the body’s normal wound-healing process. The most prevalent example is diabetes mellitus, presenting in 10.5% and 8.5% of the US and Europe populations respectively [8,9] and over 422 million individuals worldwide [10]. It is estimated that 19-34% of diabetes patients will develop foot ulcers as a result of untreated wounds, 20% of which lead to lower extremity amputations [11].
The complex wound healing process involves hemostasis, inflammation, growth, re-epithelialization, and remodeling [12], as well as regeneration of the damaged extracellular matrix (ECM). The ECM provides structural support for skin regeneration and modulates cell adhesion, proliferation, migration, and survival [13,14]. A crucial step in wound healing is the formation of a scaffold, which is later replaced by a scar, as well as the biochemical and mechanical signals required for the subsequent steps of wound healing, e.g., cell recruitment and angiogenesis [15]. Hyperglycemia, as seen in diabetes patients, disrupts the normal wound healing process by modifying ECM elasticity, thereby altering normal cell-ECM interactions and signaling [16,17]. The ensuing compromised cell migration, proliferation, and contraction induce a prolonged inflammatory phase, delayed healing process, and compromised neurovascular function, resulting in chronic wounds, such as diabetic foot ulcers18.
Autologous therapies may be ideal for accelerating wound closure in hard-to-heal wounds. Examples of such treatments include myocutaneous flap closure in diabetic foot ulcers and split-thickness graft closure. Using autologous tissue accelerates healing while avoiding patient rejection, which can occur in xenografts, allografts, and synthetic grafts[19,20]. Furthermore, the autologous platelet-rich plasma (PRP) is used as an adjuvant to grafts and promotes wound healing by providing a scaffold and growth factors [21,22]. However, those therapies require surgical expertise or special equipment and must be performed in a hospital setting or a medical clinic.
ActiGraft (RedDress Ltd., Pardes-Hanna, Israel), an autologous whole blood clot (AWBC) therapy, is a bedside treatment created from the patient’s peripheral blood without the need for special equipment or specific medical expertise. Patient blood is activated with coagulation agents to accelerate clot formation and applied into the wound to provide a fibrin scaffold that promotes wound re-epithelialization [23]. This technology has proven safe and effective in treating hard-to-heal cutaneous wounds [24-29].
The blood clot created contains all the factors required for efficient wound repair and has been shown to reduce wound size, increase angiogenesis, and expedite wound closure [24–26]. In addition, AWBC protects the wound from infection, provides a temporary ECM, and modulates the immune response.
The normal wound healing process comprises three main overlapping phases, inflammation, proliferation, and remodeling. The inflammatory stage is required for controlling bleeding, preventing infection, removing apoptotic cells, supporting cell proliferation, and tissue regeneration [30,31]. In acute wounds, macrophages play a crucial role in the wound healing process when in the initial phases of inflammation, the macrophages are in a pro-inflammatory, M1, phase and transition to the pro-healing M2 phenotype, allowing the proliferative phase to take place [30,32,33]. However, if this transition is inefficient, there will be elevated pro-inflammatory signaling, resulting in delayed healing [34]. A proposed mechanism of action of AWBC therapy is directly providing an environment that promotes this M1 to M2 macrophage switch [35]. This AWBC product provides a functional ECM, protects the wound, and facilitates the healing process. In this case series, we show the efficacy of ActiGraft in treating complicated chronic wounds associated with various comorbidities.
Material and Method
Patients
Patients with multiple comorbidities, who failed several previous treatments, and exhibiting complex wounds with exposed bone or tendon, signed the informed consent agreeing to use their medical history data, demographics, and wound characteristics, including wound images.
Procedure
Prior to ActiGraft application, all wounds underwent complete debridement of the wound tissue, bacterial balance, and moisture balance. To clear necrotic tissue, debridement was performed using surgical enzymatic, autolytic, or mechanical methods. For the ActiGraft blood clot formation, 15 mL venous blood was drawn into sterile Acid Citrate Dextrose adenine (ACDA) vacuum tubes and activated ex vivo using calcium gluconate and kaolin. Following the procedure, the blood was allowed to clot for 5-8 min, with up to 15 min in patients that are taking anticoagulant drugs. Once a clot was formed it was removed gently from the coagulation mold and attached to the wound bed using steri-strips. A non-adherent pad was placed over the clot and covered with a secondary dressing. ActiGraft was applied weekly at a point of care. Dressing changes were performed weekly as needed.
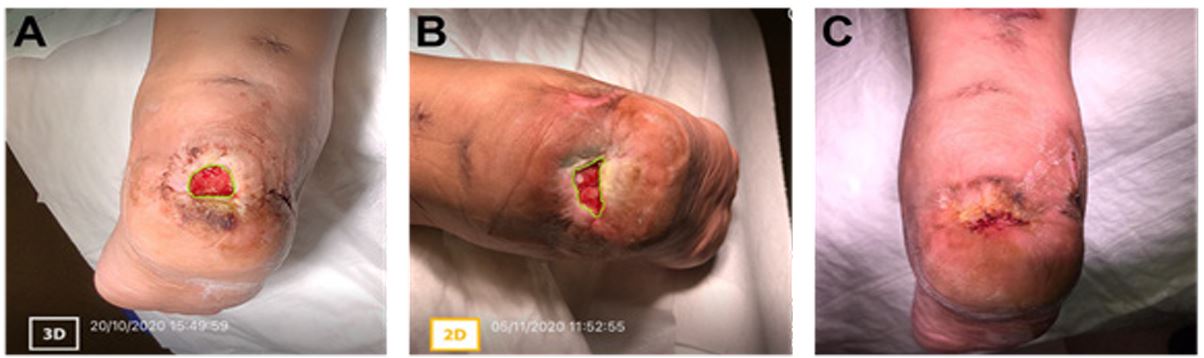
Figure 1: Case study 1: 50-year-old female with a past medical history of deep vein thrombosis and lymphedema
(A) Prior to the application of ActiGraft, the right heel ulcer measured approximately 2.2 cm2 consisted of a granular wound bed with islands of fibrotic tissue, peri-wound maceration, undermining, epibole formation, and skin induration. (B) Two weeks after ActiGraft application, the ulcer was reduced to approximately 1.3 cm2. An increase in granulation tissue and a decrease in maceration was noted. Evidence of re-epithelialization and further coalescence of wound edges was present. (C) At this time, it was determined that continued standard of care treatment was sufficient for complete wound healing
Case study 1:
A 50-year-old female with a past medical history of deep vein thrombosis (DVT) and lymphedema presented with a chronic non-healing ulcer in the posterior aspect of the right heel. The patient had undergone different standard-of-care treatment options ranging from compression, mechanical debridement, previous bi-layer skin graft application, and frequent dressing changes. However, these treatments failed to progress the wound toward healing.
Prior to ActiGraft application, the 2.2 cm2 right heel ulcer consisted of a granular wound bed with islands of fibrotic tissue, peri-wound maceration, and skin induration (Figure 1A). Undermining and epibole presence was noted. ActiGraft was applied once and kept intact for two weeks. increased coalescence of wound edges (Figure 1B). At this time, it was determined that continued standard of care treatment was sufficient for complete wound healing. (Figure 1C)
Case study 2:
An 85 year–old female with a past medical history of breast cancer to the left breast presented with a non-healing wound following surgical intervention that had been present for approximately 18 months. Biopsies did not detect any additional cancerous cells, and the affected area was treated with local wound care. Prior to the first application of ActiGraft, the wound bed appeared to be fibrogranular (mostly fibrotic tissue) with small islands of necrosis Irregularity of the wound edges was noted, alongside maceration and epibole formation. Mild peri-wound erythema was present. The wound measured approximately 29.5 cm2 (Figure 2A). ActiGraft was applied to the wound bed weekly to promote wound healing. After the first application, there was a notable improvement to the wound bed that appeared fibrogranular with an increase in granular tissue. Furthermore, there was a decrease in maceration and erythema in the peri-wound area (Figure 2B). With the continual weekly application of ActiGraft, increased granulation was noted, indicating improved wound healing. Re-epithielialization was also seen, and the wound showed increased healthy soft tissue and skin (Figure 2C-D). Following the last application of the autologous blood clot, the wound size had significantly reduced from 29.5 cm2 to 4.2 cm2, and continued granulation of the wound site was observed. The affected area exhibited signs of scarring, consistent with reduced skin integrity due to antineoplastic treatment agents. The complete eradication of necrotic tissue following treatment indicated the completion of the wound healing phases (Figure 2E).
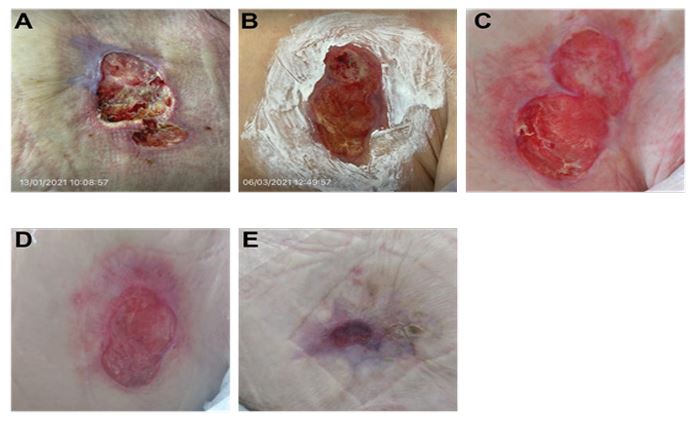
Figure 2: Case study 2: 85 year–old female with a past medical history of breast cancer presented with a non-healing wound following surgical intervention that had been present for 18 months.
(A) Prior to the first application of ActiGraft, the wound bed appeared to fibrogranular (mostly fibrotic tissue) with small islands of necrosis. Irregularity of the wound edges, alongside maceration and epibole formation was observed, as was mild peri-wound erythema. The wound measured approximately 29.5 cm2. (B) After the first application, there was a notable improvement to the wound bed, which was fibrogranular with an increase in granular tissue. There was a decrease in maceration and erythema of the peri-wound area. (C-D) Continued weekly application of ActiGraft resulted in increased granulation and re-epithelialization of the wound bed, exhibiting healthy soft tissue and skin. (E) Following the final ActiGraft application, wound size had significantly reduced from 29.5 cm2 to 4.2 cm2. Continuous granulation and re-epithelialization were noted, as were the eradication of necrotic tissue and scarring.
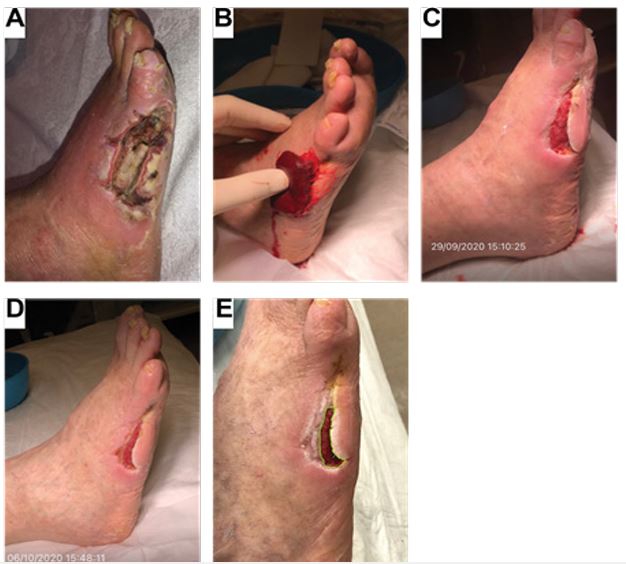
Figure 3: Case study 3: 76-year-old male with a past medical history of peripheral arterial disease and gangrenous ulcer.
(A) Chronic gangrenous ulcer measuring approximately 15 cm2 with a fibronecrotic wound bed and ischemic changes noted to the 5th digit. Cellulitis changes were noted to the foot and undermining and tunneling of the ulcerative site. (B) Application of ActiGraft onto the ulcerative site. (C) Following one week, there was Improvement of the ulcerative site with a reduction of wound bed size to approximately 5 x 2 cm. There was a decreased fibronecrotic wound bed with increased granulation to the ulcerative site and re-epithelialization of the peri-wound area/edges. There was no tunneling and a reduction in undermining. (D) Following the second treatment, there was continued healing of the ulcerative site, which measured approximately 4 x 1.7 cm. Reduced undermining noted with continued re-epithelialization of the surrounding wound edges was observed. (E) During follow-up, there was increased re-epithelialization of the peri-wound area with continuous granulation of the wound site. The ulcerative site measured approximately 2.9 x 1.1 cm.
Case study 3
A 76-year-old male with a past medical history of peripheral arterial disease (PAD) presented with a necrotic ulcer to the lateral aspect of the 5th digit following a previous gangrenous ulcer and secondary to metatarsal head resection of the 5th digit. Due to the prior PAD diagnosis, the patient required revascularization but refused vascular treatment. Limited vascular perfusion to the ulcerative site relieved the chronic nature of the ulcer. Prior to the first application of ActiGraft, the wound appeared chronic with gangrenous and ischemic changes. The ulcerative site measured approximately 15 cm2 with a fibronecrotic wound bed (Figure 3A). Cellulitic changes were noted to the foot with noted undermining and tunneling. The patient underwent two applications of the autologous blood clot applied a week apart (Figure 3B). Following the first application, there was an improvement in the ulcerative site, and the wound bed was reduced to approximately 5 x 2 cm (Figure 3C). Furthermore, there was a decrease in the fibronecrotic wound bed with increased granulation to the ulcerative site and re-epithelialization of the peri-wound area/edges. Following the second application, there was sustained healing of the ulcerative site with a reduction in size to approximately 4 x 1.7 cm, reduced undermining, and continued re-epithelialization of the surrounding wound edges (Figure 3D). Follow-up visits showed increased re-epithelialization of the peri-wound area with continuous granulation of the wound site. The ulcerative site measured approximately 2.9 x 1.1 cm (Figure 3E).
Case study 4
A 40-year-old female with a past medical history of meningocele (previous procedure for correction) and Charcot neuroarthropathy presented to the clinic following the development of an ulcer to the lateral aspect of the right foot. Offloading via a Charcot Restraint Orthotic Walker (CROW) boot was used, and the patient underwent a reconstructive pedal surgery to treat osseous and joint destruction and prevent further breakdown of the soft tissue and the spread of infection. Although various wound care modalities (i.e., multiple debridements, Medical-honey, collagen, hyaluronic acid, Negative Pressure Wound Therapy (NPWT), and PRF (platelet-rich fibrin)) were tried, granulation and re-epithelialization of the wound bed were not achieved. Prior to the first application of ActiGraft, the ulcer measured approximately 2.8 cm2 and exhibited a fibrogranular wound bed (Figure 4A).
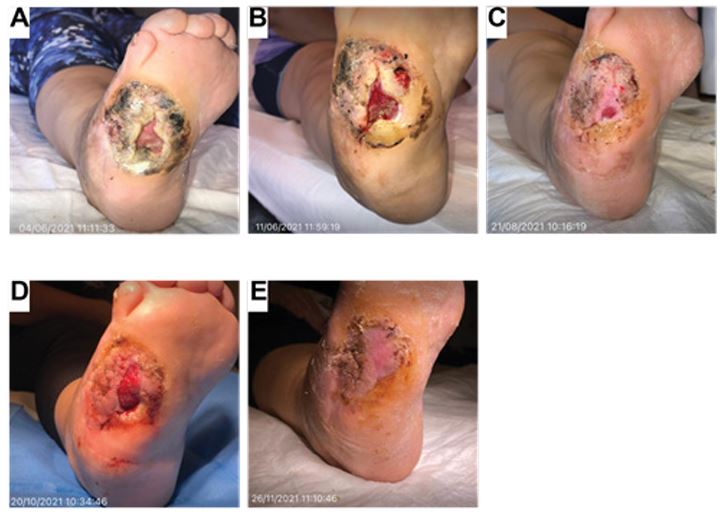
Figure 4: Case study 4: 40-year-old female with a past medical history of meningocele and Charcot neuroarthropathy
(A) Ulcer measured approximately 2.8 cm2 exhibiting a fibrogranular wound bed, limited to the subcutaneous layer. A large peri-wound hyperkeratotic rim with necrotic areas was present. Undermining and epibole were noted in the ulcerative lesion. (B) One week after the first treatment, there was a decrease in fibrotic tissue to the wound bed and in peri-wound hyperkeratotic rim necrosis. Initiation of granulation of the wound bed causing contraction of the wound edges and reduction of the epibole was observed. (C) After the second treatment, there was significant granulation and re-epithelialization of the wound bed, with the ulcer measuring approximately 1 cm2. There was the complete eradication of the epibole and continued decrease of necrosis to the hyperkeratotic rim. (D) After the tenth treatment and a two-month pause of treatment, there was a decrease in the peri-wound hyperkeratotic rim and an increase in wound size from approximately 1 cm2 to approximately 3 cm2. The ulcer consisted of a granular wound bed with no undermining or epibole present. (E) After the 14th application, complete wound healing and coalescence of wound edges were clinically observed. Adequate re-epithelialization of the wound bed was noted with minimal hyperkeratotic wound edges.
A large peri-wound hyperkeratotic rim with necrotic areas was also present, as were undermining and an epibole in the ulcerative lesion. Prior to ActiGraft treatment, the wound was thoroughly debrided to ensure a clean wound bed. Two weeks after ActiGraft application, there was a decrease in fibrotic tissue and necrosis, noted in the peri-wound hyperkeratotic rim, and initiation of granulation of the wound bed, causing contraction of the wound edges (Figure 4B). The ulcer was treated weekly with ActiGraft and exhibited a decrease in wound size, granulation of the soft tissue deficit, and a reduction of necrotic tissue. On week 10, there was significant granulation and re-epithelialization of the wound bed, and the ulcer measured approximately 1 cm2. A continued decrease of necrosis in the hyperkeratotic rim was observed (Figure 4C). Following the tenth application, the treatment was paused for two months due to the patient’s travels, which resulted in an increase in wound size; however, the decrease in the peri-wound hyperkeratotic rim continued throughout this time (Figure 4D). The ulcer consisted of a granular wound bed with no undermining. An additional four applications were performed, for a total of 14 applications. During the final treatment, complete wound healing and coalescence of wound edges were clinically observed (Figure 4E). Adequate re-epithelialization of the wound bed was present with minimal hyperkeratotic wound edges.
Discussion
Chronic wounds, in which the wound remains open for over a month, are a significant cause of patient suffering, affecting millions annually. In addition, a large percentage of chronic wounds appear in patients suffering from comorbidities4, which disrupts the normal wound healing process, causing a considerable burden on the health system.
In this case series, we present ActiGraft treatment regimen of complex open wounds in patients suffering from severe comorbidities, which are known to have a negative impact on the natural wound healing cascade, directly affecting the patient's ability to achieve wound healing, even following prolonged SOC treatment.
Venous leg ulcer
Venous leg ulcer (VLU) is attributed to venous hypertension, which induces structural changes to the vascular walls, increasing permeability [36]. In addition, a local heightened pro-inflammatory activation slows the body’s wound-healing response [37]. As a result, only 60% of wounds heal by 12 weeks, and 75% recur within 3 weeks [38].
Cancer-related ulceration
Ulcers can occur in cancer patients due to either fungating tumors, in which malignant cells infiltrate the skin and cause it to break down [39], or following radiotherapy treatment. Radiotherapy damages healthy cells in addition to cancerous ones. Therefore, there are significant side effects, including compromised wound healing due to skin atrophy, soft tissue fibrosis, and microvascular damage [40]. These radiation-induced structural changes are likely a result of a perturbation of several cellular functions required for wound healing, such as proliferation, inflammation, and remodeling [41]. Furthermore, non-healing radiation ulcers may form as a result of a combination of radiation-induced DNA mutations, microvascular damage, and fibrosis [40].
Gangrene
Gangrene is the death of body tissue due to a lack of blood flow (dry gangrene) or a severe bacterial infection (wet gangrene). Treatment involves removing dead tissue, treating and preventing the spread of infection, and diagnosing and treating the underlying condition that caused the gangrene. Tissue removal forms an open wound, whose closure may be compromised due to decreased vascularization of the affected area [42].
Charcot arthropathy
Charcot arthropathy (CN) is a chronic destructive disease of the bone structure and joints in patients with neuropathy, often associated with diabetes mellitus [43]. Up to 35% of individuals diagnosed with diabetes and neuropathy suffer from CN [44]. These structural deformities can lead to the formation of pedal ulcers, which require amputations in 15-25% of diabetic patients suffering from peripheral neuropathy and are associated with a significant increase in mortality compared to patients without ulcers [45].
During wound healing, binding to the ECM stimulates macrophages to transition from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype [30,32,33]. This attachment and differentiation upregulate platelet-derived growth factor (PDGF) secretion, inducing the wound healing’s proliferative phase. Growth factors regulate the ECM by stimulating cells to increase the production of ECM components, thereby creating a stronger scaffold for the adhesion and regulation of chemokines and mediators [46,47]. The ECM, growth factors, and newly attracted chemokines and mediators provide granulation and angiogenesis to achieve tissue regeneration and wound closure [48]. However, in chronic wounds, macrophages remain in their M1 phase, and the wound healing process remains stalled in the inflammatory phase [34]. An efficient treatment that would arrest the inflammatory stage has great potential in treating complex chronic wounds.
AWBC therapy promotes wound closure by providing a temporary ECM scaffold and recruiting the growth factors and other components required for healing [14]. Furthermore, AWBC therapy has been proposed to contain and attract many growth factors required for wound healing, including PDGF, fibroblast growth factor (FGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), and transforming growth factor (TGF) [19].
ActiGraft treatment was found to initiate the wound healing cascade and restore the conditions allowing the wound to progress toward healing. ActiGraft creates a supportive surrounding in the wound area and is suggested to attract growth factors and cytokines crucial to the healing process. ActiGraft also creates a mechanical barrier, reducing the risk of infection and bacteria bioburden.
Conclusion
In this case study, we show that ActiGraft enhances the healing of complex and chronic wounds in patients suffering from comorbidities in an efficient and timely manner. ActiGraft acts as an ECM protective scaffold, promoting the healing process by inducing the transition from the inflammatory to the proliferative phase. The major advantage of using AWBC therapy is the administration of a blood clot that includes all the necessary components for an efficient wound healing process while protecting the wound from infection. Furthermore, ActiGraft can be applied as an outpatient procedure, further reducing hospital costs and providing safe and effective wound healing treatment.
Acknowledgement
The author of this paper would like to thank Chinenye D. Wachuku, D.P.M for her help in writing this paper
References
- Järbrink K, Ni G, Sönnergren H, et al. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev. 2017; 6(1).
- Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol. 2019; 29: 8-15.
- Wicke C, Bachinger A, Coerper S, Beckert S, Witte MB, Königsrainer A. Aging influences wound healing in patients with chronic lower extremity wounds treated in a specialized wound care center. Wound Repair and Regeneration. 2009; 17(1): 25-33.
- Gould L, Abadir P, Brem H, et al. Chronic Wound Repair and Healing in Older Adults: Current Status and Future Research. Wound Repair Regen. 2015; 23(1): 1.
- Nussbaum SR, Carter MJ, Fife CE, et al. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health. 2018; 21(1): 27-32.
- Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health-care providers in Europe. 2013 ;18(4): 154-161.
- Lindholm C, Searle R. Wound management for the 21st century: combining effectiveness and efficiency. Int Wound J. 2016; 13 Suppl 2(Suppl 2): 5-15.
- Diabetes Statistics | NIDDK. Accessed November 12, 2022. https://www.niddk.nih.gov/health-information/health-statistics/diabetes-statistics
- Tamayo T, Rosenbauer J, Wild SH, et al. Diabetes in Europe: an update. Diabetes Res Clin Pract. 2014; 103(2): 206-217.
- Diabetes. Accessed November 12, 2022. https://www.who.int/news-room/fact-sheets/detail/diabetes
- Edmonds M, Manu C, Vas P. The current burden of diabetic foot disease. J Clin Orthop Trauma. 2021;17:88-93.
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev. 2019; 99: 665-706.
- Diller RB, Tabor AJ. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics. 2022; 7(3): 87.
- Snyder RJ, Schultz G, Wachuku C, Rashid AM, Ead JKK. Proposed Mechanism of Action of Topically Applied Autologous Blood Clot Tissue: A Quintessential Cellular and Tissue Based Therapy. J Am Podiatr Med Assoc. Published online October 13, 2020.
- Rausch MK, Parekh SH, Dortdivanlioglu B, Rosales AM. Synthetic hydrogels as blood clot mimicking wound healing materials. Prog Biomed Eng (Bristol). 2021; 3(4).
- Qing C. The molecular biology in wound healing & non-healing wound. Chin J Traumatol. 2017; 20(4): 189-193.
- Huang Y, Kyriakides TR. The role of extracellular matrix in the pathophysiology of diabetic wounds. Matrix Biol Plus. 2020; 6-7: 100037.
- Davis FM, Kimball A, Boniakowski A, Gallagher K. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr Diab Rep. 2018; 18(1).
- Snyder RJ, Driver V, Cole W, et al. Topical autologous blood clot therapy: an introduction and development of consensus panel to guide use in the treatment of complex wound types. Wounds. 2022; 34(9): 223-228.
- Yammine K, Assi C. A Meta-Analysis of the Outcomes of Split-Thickness Skin Graft on Diabetic Leg and Foot Ulcers. International Journal of Lower Extremity Wounds. 2019; 18(1): 23-30.
- Verma R, Kumar S, Garg P, Verma YK. Platelet-rich plasma: a comparative and economical therapy for wound healing and tissue regeneration. Cell Tissue Bank. Published online 2022.
- Ramos-Gonzalez G, Salazar L, Wittig O, Diaz-Solano D, Cardier JE. The effects of mesenchymal stromal cells and platelet-rich plasma treatments on cutaneous wound healing. Arch Dermatol Res. Published online 2022.
- Serena TE, Kushnir I, Kushnir A, Yaakov RA, Eckert KA. The safety of an autologous whole blood clot product applied to full thickness dermal wounds in a porcine model for up to 18 days. Published online 2019.
- Naude L, Idensohn P, Bruwer F, et al. An observational pilot study to collect safety and efficacy data on wound care using whole blood clot technology on hard-to-heal wounds. Wounds International Journal. 2021; 12(2).
- Kushnir I, Kushnir A, Serena T, Garfinkel D. Efficacy and Safety of a Novel Autologous Wound Matrix in the Management of Complicated, Chronic Wounds: A Pilot Study. Wounds. 2016; 28(9).
- Snyder R, Kasper M, Patel K, et al. Safety and Efficacy of an Autologous Blood Clot Product in the Management of Texas 1A or 2A Neuropathic Diabetic Foot Ulcers: A Prospective, Multicenter, Open Label Pilot Study. Wounds. 2018; 30(7).
- Gurevich M, Wahab N, Wachuku C, Ead KJ, Snyder RJ. ActiGraft Treatment in Complex Wounds with Exposed Structure - A Case Series. USA Ann Rev Resear. 2021; 7(1).
- Williams M, Davidson D, Wahab N, Hawkins J, Wachuku CD, Snyder R. Innovative treatment utilizing an autologous blood clot for diabetic foot ulcers. Wounds. 2022; 34(7).
- Landau Z, Whitacre KL, Leewood C, Hawkins J, Wachuku CD. Utilization of a topical autologous blood clot for treatment of pressure ulcers. Int Wound J. Published online 2022.
- Eming SA, Krieg T, Davidson JM. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. Journal of Investigative Dermatology. 2007; 127(3): 514-525.
- Koh TJ, DiPietro LA. Inflammation and wound healing: The role of the macrophage. Expert Rev Mol Med. 2011; 13: e23.
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: A cellular perspective. Physiol Rev. 2019; 99(1): 665-706.
- Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. International Journal of Molecular Sciences. 2017; 18(7): 1545.
- Verdolino DV, Thomason HA, Fotticchia A, Cartmell S. Wound dressings: curbing inflammation in chronic wound healing. Emerg Top Life Sci. 2021; 5(4): 523-537.
- Snyder RJ, Lantis J, Kirsner RS, Shah V, Molyneaux M, Carter MJ. Macrophages: A review of their role in wound healing and their therapeutic use. Wound Repair Regen. 2016; 24(4): 613-629.
- Xie T, Ye J, Rerkasem K, Mani R. The venous ulcer continues to be a clinical challenge: an update. Burns & Trauma. 2018; 6(1): 1-7.
- Ligi D, Mosti G, Croce L, Raffetto JD, Mannello F. Chronic venous disease – Part I: Inflammatory biomarkers in wound healing. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2016; 1862(10): 1964-1974.
- Fernandes Abbade LP, Lastória S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol. 2005; 44(6): 449-456.
- Tandler S, Stephen-Haynes J. Fungating wounds: management and treatment options. 2017; 26(12): S6-S14.
- Dormand EL, Banwell PE, Goodacre TEE. Radiotherapy and wound healing. Int Wound J. 2005; 2(2): 112-127.
- Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG. Wound healing after radiation therapy: review of the literature. Radiat Oncol. 2012; 7(1).
- Buttolph A, Sapra A. Gangrene. StatPearls. Published online August 8, 2022. Accessed November 16, 2022. https://www.ncbi.nlm.nih.gov/books/NBK560552/
- Dardari D. An overview of Charcot’s neuroarthropathy. J Clin Transl Endocrinol. 2020; 22: 2214-6237. doi:10.1016/J.JCTE.2020.100239
- Rosskopf AB, Loupatatzis C, Pfirrmann CWA, Böni T, Berli MC. The Charcot foot: a pictorial review. Insights Imaging. 2019; 10(1).
- Yammine K, Boulos K, Assi C, Hayek F. Amputation and mortality frequencies associated with diabetic Charcot foot arthropathy: a meta-analysis. Foot and Ankle Surgery. Published online August 13, 2022.
- Chang M. Restructuring of the extracellular matrix in diabetic wounds and healing: A perspective. Pharmacol Res. 2016;107:243-248.
- Zubair M, Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev Endocr Metab Disord. 2019; 20(2).
- Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009; 17(2): 153-162.

