Review Article - Volume 3 - Issue 3
Biobioequivalence of Immediate Release-Glibenclamide 5mg Tablets: A Comparative Study in Healthy Volunteers
Abdelkarim M; Abdelkarim; Murtada A. Oshi*
Department of Pharmaceutics, Faculty of Pharmacy, Omdurman Islamic University, Omdurman, Sudan.
Received Date : April 15, 2023
Accepted Date : May 12, 2023
Published Date: May 29, 2023
Copyright:© Murtada A. Oshi 2023
*Corresponding Author : Murtada A. Oshi, Assistant Professor, Department of Pharmaceutics, Faculty of Pharmacy, Omdurman Islamic University, Omdurman, Sudan.
Email: oshiphar@yahoo.com
DOI: Doi.org/10.55920/2771-019X/1448
Abstract
Background: Glibenclamide is a sulfonylurea oral hypoglycemic drug, which has long been in clinical use for diabetes mellitus.
Objectves: This study aims to assess the bioequivalence (pharmacokinetics and antihyperglycemic effect) in healthy volunteers under fasting conditions of three locally manufactured generics of glibenclamide 5 mg tablets (Aminil®, Y and Z) available in Sudan. Similarly, the originator glibenclamide 5 mg tablets (Euglucon®) was assessed and used as a reference product.
Methods: Nine healthy subjects were given a 5 mg dose of Euglucon® (Roch) or Aminil® (Amipharma lab) tablets. Blood samples were collected at different time intervals analyzed for the glucose concentration. Also, the pharmacokinetic parameters such as the maximum mean serum concentration (Cmax), the mean time to maximum serum concentration (Tmax), the area under the concentration versus time curve (AUC) and the drug clearance (CL) were recorded. The mean glucose concentration was also determined in different time intervals.
Results: The obtained results showed no significant difference in the mean Tmax between the tested items. However, the mean Cmax was significantly higher (p<0.001) for Aminil® tablets (456 ng/mL) compared to Euglucon® tablets (291 ng/mL). Similarly, the mean AUC0-8h was significantly higher (p<0.001) with Aminil® tablets (1915 ng/mL.h) compared to Euglucon® tablets (1163 ng/mL.h). After 8 h, the drug clearance of 0.0430 L/Kg.h for Euglucon® tablets and 0.0260 L/Kg.h for Aminil® tablets. Both products lowered the mean glucose concentrations after 8 h, 99 mg/dL for Euglucon® tablets and 98 mg/dL for Aminil® tablets.
Conclusions: Collectively, in this study Aminil® tablets showed pharmacokinetic parameters and anti-hyperglycemic effect in healthy volunteer similarly to that of Euglucon® tablets. Therefore, Aminil® tablets is bioequivalent to the Euglucon® tablets.
Clinical Relevance: This approach can help clinical pharmacist’ to improving their drug dispensing practice and skill that ultimately will improve patients’ quality of life.
Keywords: Diabetes mellitus, hyperglycemic effects, glibenclamide, pharmacokinetics, tablet
Introduction
The physical, chemical and biological qualities of medicines are considered as major aspects. This is due to the drug pharmacological action in treating the target disease would be largely affected by the quality of medicines and pharmaceuticals [1-3]. In Sudan as like other Sub-Saharan countries in Africa, the regulation of the medicines and maintaining their quality standard is governed mainly by the Federal Department of Pharmacy. The role of this department is to ensure that all the medicines (imported items and local items) and used by the patients should be fulfilled certain standards of quality standards, safety and efficiency [4]. Beside the department is also responsible for making the medicines are available in affordable prices for all patients. To implement all these important requirements, vigorous systems of medicines registrations and pharmacy premises licensing are currently used [4,5].
In Sudan, the needs for medicines has largely increased during the last two decades. This might be attributed to the factors such as fast growing of the country’s population and improving in the health supplies systems [4]. However, the high needs for the medicines by the Sudanese’s people has put a burden to the Federal Departments of Pharmacy in regarding testing of their qualities and effectiveness [4-6]. As the consequence, variables in the clinical effectiveness and decrease of user's confidence (pharmacists, physicians and patients) have been reported in recent years in the local pharmaceutical markets in Sudan. The Federal Departments of Pharmacy seeks for primary criteria to ensure the best quality of medicines and pharmaceuticals to user and to prevent sub-standard product, which does not fall within international standards. Quality control parameters of medicines, such as dissolution time and drug potency are useful tools for maintaining consistency in batch-to-batch manufacturing. All quality control parameters are closely related to each other and have effect on drug pharmacokinetic profiles, bioavailability and finally in drug pharmacological actions [7,8].
Glibenclamide is a potent oral hypoglycemic agent belonging to the sulphonylurea group. It is a slightly acidic drug with high lipophilicity. It is frequently prescribed for the treatment of late-onset (non-insulin dependent) diabetes mellitus. However, the commercialized glibenclamide products, such as Euglucon® are slowly and incompletely absorbed by the human gastrointestinal tract (GIT). This would be made the drug to have poor bioavailability after the oral administration. Many factors known to influence the bioavailability of glibenclamide such as the presence of food in the GIT and the physical form of the drug product [9].
The objective of this study is to ensure conformity of quality of glibenclamide tablets available in the Sudan. To fulfil these objective, the glibenclamide tablets 5 mg from four different companies (imported and locally manufactured items) were investigated for their pharmacokinetics and anti-hyperglycemic effect in healthy volunteer subjects.
Materials and methods
Study design
This was a single-center comparative bioequivalence, open-label, randomized, and single-dose study under fasting conditions. The study consisted of one single-dose administrations of glibenclamide immediate release tablet (with water followed by taking standard breakfast after 1.5 h of drug intake).
The study was open label. Because comparative bioequivalence studies involve a comparison of pharmacokinetic profiles and antihyperglycemic effect, which are not subjective measurements, blinding was not deemed necessary for this study. The clinical study protocol, any related associated documents, and informed consent forms were reviewed and approved by an independent ethics committee at University of Khartoum, Sudan.
Study population
Volunteers enrolled in this study were members of the community at large. Before entering the study, all volunteers were undergoing for medical history checkup, physical examination and biochemical testing. The group of subjects taking part in the experiments were comparable in age, height, and weight and demographic data (race and ethnicity). All subjects were forbidden from vigorous work for three days before the beginning of the study. All participating volunteers were judged eligible for the study when assessed against these criteria.
Study Procedures
At the study day, according to the randomization scheme, volunteers were administered a single oral dose of either the test glibenclamide, as 5 mg unit-dose immediate release-tablet with 150 mL distilled water followed by taking standard breakfast after 1.5 h of drug intake or reference glibenclamide tablet (Euglucon®). Then, indwelling catheter was inserted to each volunteers’ vein and 10 mL of blood samples were taken from each subject.
It is worth to mention that the other glibenclamide tablets brands (Y and Z) were excluded from the further studies because they failed to pass preliminary quality control tests (weight variation, friability, hardness, disintegration time, content uniformity and drug dissolution time). The results obtained from these tests were not shown.
Analytical Methods
Blood was collected and allowed to clot at room temperature for at least 30 min. The serum was then separated by centrifugation and harvested. The serum samples were then stored at-20°C until assayed. The serum concentration of total glibenclamide was determined by using high performance liquid chromatography (HPLC) method [10].
Pharmacokinetic study
The plasma glibenclamide concentration was used to determine the following pharmacokinetic parameters: the maximum drug concentration in plasma (Cmax); time to reach Cmax (Tmax); area under the concentration area curve (AUC) and clearance (CL).
Anti-hyperglycemic effect
Serum samples were taken at 0, 1, 2, 3, 4, 5, 6, 7 and 8h from the subjects. All samples were subjected to clot retraction and centrifuged at 3500 rpm for 15 min. lastly, the serum samples were divided into two portions, one used immediately for glucose analysis by hexokinase method, and the other kept at - 20 ºC for glibenclamide analysis by special HPLC technique. The concentrations of glibenclamide for the two brands were determined by HPLC [10].
Statistical analysis
The statistical significance of weight and hardness of tablet of different brands was carried out with SPSS-Computer software (Version 15.0). At 95% confidence interval, 2-tailed p value less than or equal to 0.05 were considered significant.
Results and Discussions
Participating volunteer
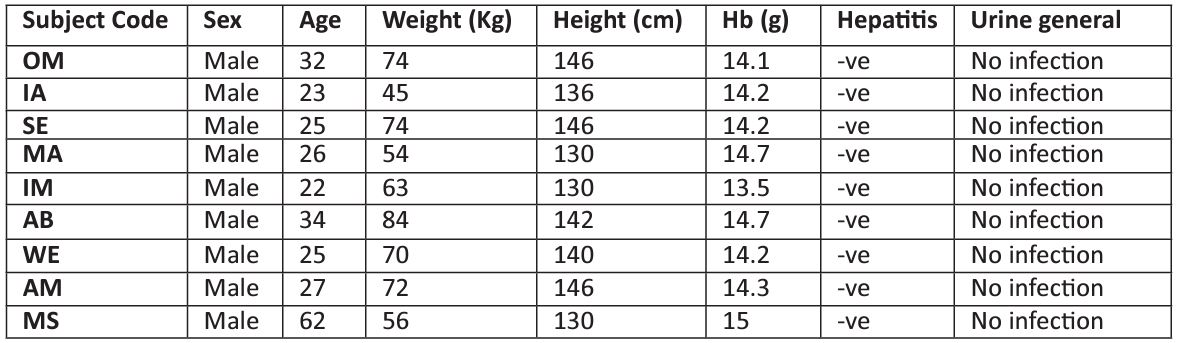
Table 1 shows the characteristics of nine subjects in the treatment groups and biochemical and hematologic screening results. All subjects were in healthy state as the tests results were confirmed and therefore, no one was withdrawn from the study.
Table 1: The characteristic of subjects in the treatment groups.
Pharmacokinetic assessment
Table 2 shows the pharmacokinetic parameters (Cmax and Tmax) of Aminil® and Euglucon® tablets. The mean Tmax was 3 min for the both Aminil® and Euglucon® tablets. The mean Tmax when Euglucon® tablets were used was not significantly different to that of Aminil® tablets. Unexpectedly, in this study we found that the mean Cmax when Euglucon® glibenclamide 5 mg was used was significantly different to that after using Aminil® tablets. The Tmax for Euglucon® is 290 ng/mL and for Aminil® tablet is 456 ng/mL.
Table 2: Pharmacokinetic parameters (Cmax and Tmax ).
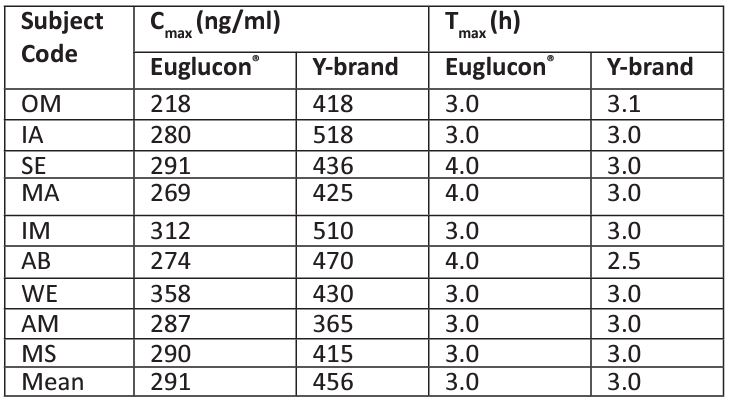
Table 3 shows the pharmacokinetic parameters (AUC and Cl) of Aminil® and Euglucon® tablets. As shown in the table, the mean AUC was significantly higher with Aminil® compared to Euglucon® tablets. On the other hands, the Cl was 0.026 L/kg/h for Aminil® tablet and 0.043 L/kg/h for Euglucon® tablet.
The in-vitro dissolution studies have demonstrated that the extent of dissolution of Aminil® tablet was significantly higher than the Euglucon® tablets (data not shown). The lower degree of solubility of Euglucon® was responsible for the apparent incomplete absorption from the GIT in the in-vivo situation compared Aminil® tablet.
The present study showed clearly the large difference in the bioavailability of the Euglucon® compared to Aminil® tablet. So the Euglucon® tablets must be formulated in lower dose as already seen in the literature. 5 mg Aminil® tablet can be taken as over dose, which may be associated with serious hazards such as hypoglycemia.
Table 3: Pharmacokinetic parameters (AUC and CL ) obtained from the 9 volunteers.
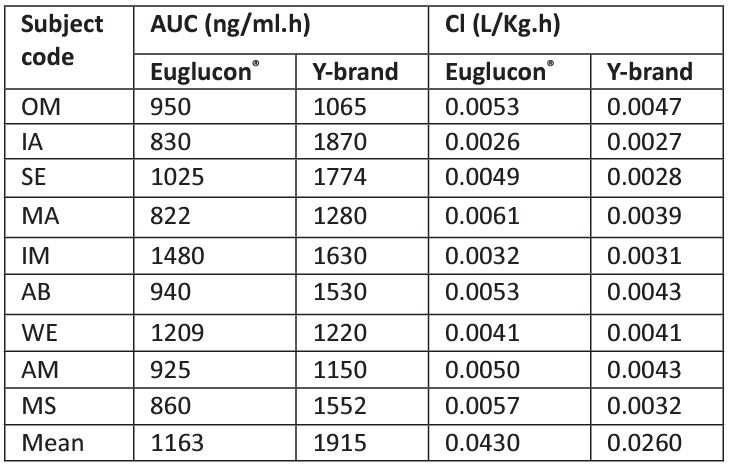
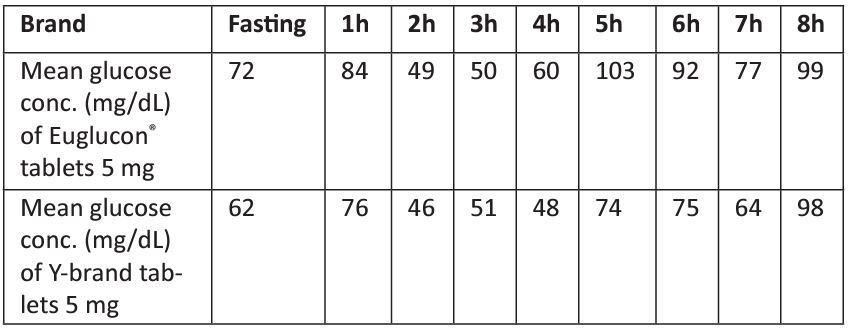
Table 4: The mean glucose concentration obtained from the 9 volunteers in different time intervals.
Antihyperglycemic effect assessment
Table 4 shows the mean glucose concentration obtained from the 9 volunteers in different time intervals. At baseline, the mean concentration of glucose was 72 mg/dL for volunteers taking Euglucon® tablets and 62 mg/dL for volunteers taking Aminil® tablet after breakfast. As shown in the table, the concentration of glucose raised to 84 mg/dL and 76 mg/dL for volunteers taking Euglucon® and Aminil® tablet, respectively. After 2 h from taking the drug, the concentration was only 49.6 mg/dL and 46 mg/dL for volunteers taking Euglucon® and Aminil® tablets, respectively. After 4h the concentration of glucose for volunteers using Aminil® tablet was 48 mg/dL and 60 mg/mL for volunteers using Euglucon® tablets.
In this study, we observed that in the elderly volunteers the action of the Euglucon® tablets were stronger than of Aminil® tablet. The glucose concentration was decreased dramatically for 62 year-old volunteer at 4 h from drug intake, and the concentration of glucose was only 32 mg/dL, and was given glucose orally to overcome the hypoglycemia.
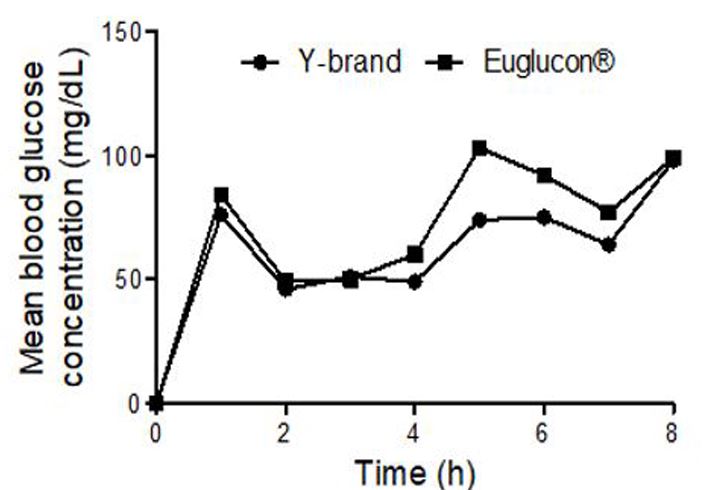
Figure 1: Mean blood concentration of glucose in serum versus time of 9 subjects taking Aminil® tablet or Euglucon® tablets
Conclusion
The present study showed clearly that there is no large difference in the bioequivalence properties (pharmacokinetic parameters and antihyperglycemic effect) between the locally manufactured immediate-release glibenclamide 5 mg item Aminil® tablet and imported item Euglucon® tablet that are available in the Sudanese pharmaceutical markets. Therefore, these two immediate release-glibenclamide 5 mg tablets can be used exchangeable as an antihyperglycemic agents for diabetic patients.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Khuluza F. In-vitro evaluation of the quality of Paracetamol and Co-trimoxazole tablets used in Malawi based on pharmacopoeial standards. Malawi Medical Journal. 2014; 26(2): 38-41.
- Dulla O, Sultana S, Shohag M. In vitro comparative quality evaluation of different brands of esomeprazole tablets available in selected community pharmacies in Dhaka, Bangladesh. BMC Research Notes. 2018; 11(1): 184-188.
- Bate R, Hess K. Assessing website pharmacy drug quality: safer than you think. PLoS One. 2010; 5(8): e12199.
- Omer AM, Ali GK. Assessment of the Substandard Drugs in Developing Countries: The Impact of the Pharmaceutical Regulations on the Quality of Medicines on the Sudanese Market Importers’ Perspective. Medical Research Chronicles. 2014; 1(3):171-181.
- Vogel RJ, Stephens B. Availability of pharmaceuticals in sub-Saharan Africa: roles of the public, private and church mission sectors. Social Science and Medicine. 1989; 29(4): 479-486.
- Van der S. The efficiency of inefficiency. Medicine distribution in South Cameroon. 1982; 16(24): 2145-2153.
- Markl D, Zeitler J. A Review of Disintegration Mechanisms and Measurement Techniques. Pharmaceutical research. 2017; 34(5): 890-917.
- Wingstrand K, Abrahamsson B, Edgar B. Bioavailability from felodipine extended-release tablets with different dissolution properties. International Journal of Pharmaceutics. 1990; 60(2): 151-156.
- Otoom S, Hasan M, Najib N. The bioavailability of glyburide (glibenclamide) under fasting and feeding conditions: a comparative study. International Journal of of Pharmaceutical Medicine. 2001; 15(3): 117-120.
- Rajendran S, Philip B, Gopinath, R, Suresh B. HPLC Method for the Estimation of Glibenclamide in Human Serum. Indian Journal of Pharmaceutical Sciences. 2007; 69(6): 1-5.

