Case report - Volume 3 - Issue 3
Rapidly progressive renal dysfunction in systemic sclerosis: Scleroderma renal crisis or…?
Pallavi Mahato; Atanu Pal*; Souvik Ghatak; Anirban Sen; Koushik Bhattacharjee; Sanjay Dasgupta
Department of Nephrology, IPGME&R, West Bengal, India.
Received Date : May 09, 2023
Accepted Date : June 13, 2023
Published Date: June 20, 2023
Copyright:© Atanu Pal 2023
*Corresponding Author : Atanu Pal, Assistant Professor, Department of Nephrology, IPGME&R, West Bengal, India.
Email: projectdratanu@gmail.com
DOI: Doi.org/10.55920/2771-019X/1464
Abstract
Systemic sclerosis is an autoimmune disorder involving skin, gastro-intestinal tract, lungs, kidneys and heart. Rapidly progressive renal dysfunction and accelerated hypertension remains the hallmark for scleroderma renal crisis in patients of Systemic sclerosis. Here we present a case of 38 year old male patient who presented with clinical signs of systemic sclerosis and unexplained renal dysfunction associated with hypertension. A provisional of Scleroderma renal crisis was expected but renal biopsy, surprisingly, revealed features suggestive of Crescentric Glomerulonephritis.
Keywords: Systemic Sclerosis; Scleroderma Renal Crisis; Rapidly Progressive Renal Failure; Autoantibodies; crescentric glomerulonephritis; infection related glomerulonephritis.
Abbreviation: SRC: scleroderma renal crisis; GN: glomerulonephritis; IRGN: infection related glomerulonephritis
Introduction
Systemic sclerosis (SS) is an autoimmune disease of unknown etiology and characterized by fibrosis and thickening of skin and systemic involvement including gastrointestinal tract, lungs, kidneys and heart [1,2]. It is more commonly seen in females. SS has an overall female to male ratio of 3:1 or greater. [3] Diffuse systemic sclerosis and limited cutaneous systemic sclerosis are the two subsets of SS based on the extent of skin and systemic involvement. Oesophageal dysmotility, pulmonary fibrosis, arterial hypertension and hypertensive heart failure are some of the systemic manifestations of SS [3]. Around 10% of the patients develop scleroderma renal crisis (SRC), a rapidly progressive oliguric renal failure due to immune mediated vascular endothelial injury [4]. But some patients rarely may have alternative pathology leading to SRC like presentation. Here we present a similar challenging case of mimicking SRC, and the role of renal biopsy in the diagnosis.
Case presentation
A 38 year old non diabetic, normotensive and euthyroid patient presented with decreased urine output and new onset hypertension for last 2 months. On further enquiry, it was found he had a history of chronic cough for last 15yrs which was not associated with any expectoration or hemoptysis. He also had a history of Raynaud’s phenomenon along with decreased oral aperture (figure 1) for last 6 yrs. But there was no history of skin tightening, dysphagia, palpitation, joint pain, skin rash or telengectesias.
On examination his blood pressure was 160/100 mm of Hg, pulse rate 84/minutes. There was pallor but no skin pigmentation or tightening. His oral aperture was markedly narrowed and did not permit more than two fingers to pass. There was no joint tenderness or swelling in his hands, no ulcers or gangrene but there was history of Raynaud’s phenomenon. On auscultation, there were B/L coarse crepitations. Rest of the physical examination was normal.
Baseline investigations revealed Hemoglobin 9.4 gm/dL, platelets 2.2 lakh/mm3 and Total count 10,100/mm3. Serum sodium was 134 mmol/L, potassium 4.2 mmol/L, urea 78mg/dL, creatinine 3.1 mg/dL. HBsag HCV HIV1 &2 were negative. Urine routine examination showed albumin 2+; 2-3 pus cell and 24 hours quantitative urine protein was 900 mg. Ultrasound of abdomen showed normal sized kidneys with slightly raised cortical echogenicity and maintained cortico-medullary differentiation. Echocardiography showed left ventricular ejection fraction of 60% no regional wall motion abnormality, no pulmonary artery hypertension. UGI endoscopy was normal. HRCT thorax shows- gross emphysematous changes with honey combing and ground glass opacities in both lungs lower zone (figure 2).
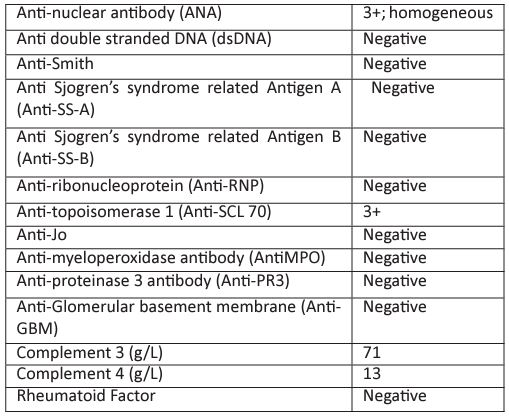
From the history and physical examination, we suspected Systemic sclerosis. In view of HRCT findings and normal echocardiography, the chronic dry cough was attributed to Interstitial Lung Disease. Immunological workup was done for the patient (TABLE 1) C3- 71.2, C4- 13.7 ANA- 3+ HOMOGENOUS; SCL 70- +++; ANCA- negative; ANTI GBM-NEGATIVE; ANTI PR3: NEGATIVE; ANTI MPO: NEGATIVE. The Raynaud’s, ILD and autoantibodies (Scl-70) confirmed the diagnosis of diffuse systemic sclerosis according to the ACR/EULAR 2013 criteria [5]. In view of the sudden rise in blood pressure and RPRF against the clinical background, a provisional diagnosis of SRC was kept. But the Renal biopsy finding revealed: (figure 3)
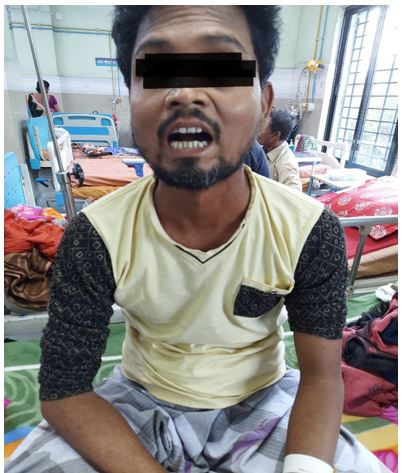
Figure 1: Small oral aperture
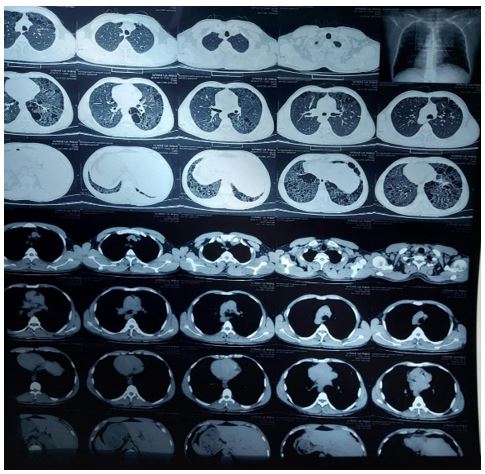
Figure 2: HRCT Thorax showing bilateral diffuse ground glass opacities.
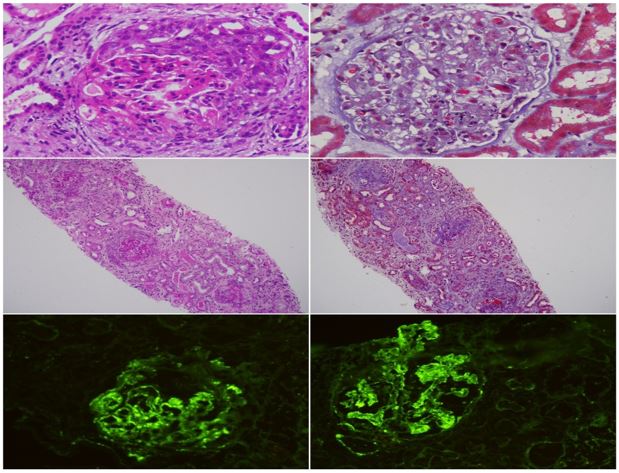
Figure 3: Renal biopsy showing circumferential cellular crescent formation, diffuse endocapillary proliferation, immunofloroscence showing full house pattern.
Table 1: Immunological workup was done for the patient.
LIGHT MICROSCOPY- Eighteen glomeruli are identified, six are globally sclerotic. Cellular crescents are observed in eight glomeruli and fibrocellular crescent in one glomerulus. Fibrous crescents are seen in two glomeruli. Glomerular basement membrane shows no spikes or double contour. Segmental endocapillary proliferation is seen. There is no necrotizing lesion. There is tubular epithelial cell injury. Interstitium shows lymphocytic infiltration. Interstitial fibrosis and tubular atrophy involve about 10-20% of the sampled cortex. Artery shows mild medial hypertrophy.
IF STUDY- 3 glomeruli are present for evaluation. The sections are stained for IgG, IgM,IgA,C3,C1q,Kappa& Lambda light chains. C3 (+3) and IgG (+2) show granular positivity on the glomerular capillary loops. No light chain restriction seen. Rest of the antisera are negative.
DIAGNOSIS-Endocapillary proliferative glomerulonephritis consistent with infection related glomerulonephritis with crescentic transformation.
Hence a diagnosis of IRGN in the background of SS was done with associated ILD. The cause for IRGN or any subtle source of infection could not be identified after doing a thorough search.
Discussion
Contrary to common belief, all renal involvement in SS is not SRC. SS can have a wide spectrum and entire range of renal involvement, SRC being the most grave and often leading to ESRD. Other renal manifestations associated with SS includes: Normotensive renal crisis; ANCA MPO associated GN, D penicillamine use associated renal disease, Proteinuria and microalbuminuria and Isolated reduction in glomerular filtration rate [6].
SRC usually manifests as RPRF in presence of accelerated hypertension. The pathogenesis of SRC is poorly understood, and probably mediated by both immunological and non-immunological pathways [7]. It usually responds to ACEi. SRC has strong association with anti polymerase 3 antibody level, Rapidly progressive skin disease, tendon friction rubs, Corticosteroid exposure (≥15 mg per day in preceding 6 months), HLA-DRB1*0407 and HLA-DRB1*1304 [8].
Normotensive SRC has worse renal overall prognosis than hypertensive SRC. It is usually attributed to the prolonged subclinical renal injury that goes undetected for a long time. Also, the clinical improvement seen in hypertensive SRC patients on addition of ACEI/ARBS is not seen in normotensive SRC patients [9].
Crescentic GN is characterized by disruption of the glomerular basement membrane leading to cellular proliferation in the Bowman's space with fibrinoid necrosis. It is of major 3 types [10]: (10% cases)Anti-glomerular basement membrane (anti-GBM) disease: characterized by circulating anti-GBM antibodies and linear deposition of antibodies along the glomerular basement membrane. (60%) Pauci-immune (anti-neutrophil cytoplasmic antibodies (ANCA)-associated GN: scanty glomerular deposits of immunoglobulin and circulating ANCA (Rest 30%) Immune complex-mediated GN: granular deposits of immunoglobulins, in which crescent formation with proliferative GN is seen like infections (HCV), auto immune disease (SLE) or other primary GN. Crescentic GN is a very uncommon cause of renal failure in SS. Glomerulonephritis associated with myeloperoxidase-specific antineutrophil cytoplasmic antibodies (ANCA) has been reported in some cases of SS [11].
20% of patients treated with D-Penicillamine develop reversible membranous glomerulonephritis and proteinuria. But use of Penicillamine is obsolete now. Mortality in such cases may go as high as 40% [12].
In up to 25% of SS patients proteinuria or albuminuria may be present, with intermediate-weight proteinuria in about 31.3% of the cases. Intermediate-weight proteinuria is a strong predictor of glomerular permeability and correlates strongly with gastrointestinal involvement [13].
Isolated reduction in glomerular filtration rate may also be seen and it correlates with vasculopathic manifestations. In pulmonary hypertension associated with SS, estimated GFR is a strong predictor of survival, with eGFR less than 60 ml/min per 1.73 m is associated with poor prognosis and mortality [14].
Infection related glomerulonephritis associated with Systemic Sclerosis is a very rare concurrence. After much such search in literature, no previous such case report was found. Our patient had a presentation same as of SRC, so we expected a biopsy finding suggestive of SRC. Our next differential was that of ANCA associated vasculitis, as it is the second most common renal pathology associated with SS. But to our surprise, we found features characteristically associated with IRGN, that is, the presence of typical endocapillary or endocapillary with mesangial proliferation with or without the presence of neutrophilic infiltration [15].
We treated this patient conservatively as steroid and ACEi have no role in IRGN. His Cr stabilised at around 2 without any need for Renal replacement therapy. For the ILD component, he was given two doses of Rituximab @ 375mg/m2 one month apart. 6 months have passed and he is having a good quality of life without any dependence on Renal replacement therapy
Hence it is not necessary that RPRF associated with hypertension in a patient of SS indicates SRC, other pathologies may exist and mimic a similar presentation [16]. So every case of unexplained renal dysfunction should be subjected to renal biopsy expecting alternative pathologies because the treatment and prognosis differs hugely depending on the underlying pathology [17].
References
- Asamoah-Odei Efu. Scleroderma Renal Crisis in a Normotensive Patient. Kidney International Reports. 2016; 1(4): 311-315.
- Akoglu Hadim.A “silent” course of normotensive scleroderma renal crisis: case report and review of the literature. Rheumatology International. 2009; 29(10): 1223-1229.
- Peoples C, Medsger TA Jr, Lucas M, Rosario BL, Feghali-Bostwick CA. Gender differences in systemic sclerosis: relationship to clinical features, serologic status and outcomes. J Scleroderma Relat Disord. 2016; 1(2): 177-240.
- Ubukata Masamitsu et al. Anticentromere Antibody-positive Scleroderma Renal Crisis Requiring Dialysis. Internal Medicine. 2018; 57(23): 3479-3483.
- Van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. Classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013; 65(11):2737-47.
- Shanmugam VK, Steen VD. Renal disease in scleroderma: an update on evaluation, risk stratification, pathogenesis and management. Curr Opin Rheumatol. 2012; 24(6): 669-76.
- Ibrahim Batal, Robyn T. Domsic, Thomas A. Medsger, Sheldon Bastacky. Scleroderma Renal Crisis: A Pathology Perspective. International Journal of Rheumatology. 2010; 2010: 7.
- Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005; 35(1): 35-42.
- Akoglu Hadim., et al. “A “silent” course of normotensive scleroderma renal crisis: case report and review of the literatur. Rheumatology International. 2009; 29(10): 1223-1229.
- Moroni G, Ponticelli C. Rapidly progressive crescentic glomerulonephritis: Early treatment is a must. Autoimmun Rev. 2014; 13(7): 723-9.
- Anders HJ, Wiebecke B, Haedecke C, Sanden S, Combe C, Schlöndorff D. MPO-ANCA-Positive crescentic glomerulonephritis: a distinct entity of scleroderma renal disease? Am J Kidney Dis. 1999; 33(4): e3.
- Derk CT, Jimenez SA. Goodpasture-like syndrome induced by D-penicillamine in a patient with systemic sclerosis: report and review of the literature. J Rheumatol. 2003; 30(7): 1616-20.
- Seiberlich B, Hunzelmann N, Krieg T, Weber M, Schulze-Lohoff E. Intermediate molecular weight proteinuria and albuminuria identify scleroderma patients with increased morbidity. Clin Nephrol. 2008; 70(2): 110-7.
- Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010; 182(2): 252-60.
- Gupta P, Gupta RK. Infection Related Glomerulonephritis (IRGN). In: Pathology of Glomerular Diseases. Springer, Singapore. 2022.
- Ramaswami A, Kandaswamy T, Rajendran T, Jeyakrishnan KP, Aung H, Iqbal M, et al. Scleroderma with crescentic glomerulonephritis: a case report. J Med Case Rep. 2008; 2: 151.
- Kidney Blood Press Res. 2020; 45(4): 532-548.

