Case report - Volume 3 - Issue 4
Systemic Burkholderia cepacia complex in a patient with ACLF associated to extreme polarization of the intestinal microbiota toward the phylum Proteobacteria: A case report and review of literature
Baltazar-Díaz Tonatiuh A¹, Moreno-Ortiz José M², González-Hernández Luz A³, Bueno-Topete Miriam R*¹
1Instituto de Investigación en Enfermedades Crónico Degenerativas, Departamento de Biología Molecular y Genómica, Centro Universitario de Ciencias de la Salud, Sierra Mojada 950, Universidad de Guadalajara, Guadalajara, México.
²Instituto de Genética Humana “Dr. Enrique Corona Rivera”, Departamento de Biología Molecular y Genómica, Centro Universitario de Ciencias de la Salud Sierra Mojada 950, Universidad de Guadalajara, Guadalajara, Jalisco, México.
³Hospital Civil de Guadalajara, Unidad Hospitalaria Fray Antonio Alcalde, Unidad de VIH, Hospital 278, Guadalajara, Jalisco, México.
Received Date : June 07, 2023
Accepted Date : July 07, 2023
Published Date: July 10, 2023
Copyright:© Bueno-Topete Miriam R 2023
*Corresponding Author : Bueno-Topete Miriam R, Instituto de Investigación en Enfermedades Crónico Degenerativas, Departamento de Biología Molecular y Genómica, Centro Universitario de Ciencias de la
Salud, Sierra Mojada 950, Universidad de Guadalajara, Guadalajara, México. Tel: (+52) (33) 1058 5200, ext. 34017,
Email: ruthmyriamtop@hotmail.com
DOI: Doi.org/10.55920/2771-019X/1494
Abstract
Burkholderia cepacia (Bcc) bloodstream infection, without bacterial peritonitis, has not been reported in cirrhosis. We report the case of a 51-year-old male inpatient with alcohol-associated cirrhosis complicated on admission, who was unresponsive to treatment and died 48 hours later. Thirty minutes before the patient’s death, stool and blood samples were taken. Albeit cultures were negative, Sanger and metagenomic sequencing of 16S-rRNA and ITS2 genes from samples revealed systemic infection dominated by Bcc, however other Proteobacteria were also identified. Interestingly, we observed that gut microbiota polarized toward Proteobacteria/Gammaproteobacteria/Enterobacteriaceae/Escherichia/Shigella, which suggests bacterial translocation as a source of systemic infection. Regarding Bcc, it is resistant to several antibiotics, hence timely and accurate molecular tests might help to improve clinical outcomes.
Keywords: Liver cirrhosis, Burkholderia, Acute-On-Chronic Liver Failure, Microbiota
Introduction
The Burkholderia cepacia complex (Bcc) is a group of aerobic Gram-negative rod-shaped bacteria, some of which are opportunistic pathogens [1]. Bcc infection has been rarely reported in patients other than cystic fibrosis. In liver cirrhosis, there are reports of SBP (spontaneous bacterial peritonitis) caused by Bcc [2]. However, to date, no study has reported the systemic presence of these bacilli in the context of decompensated alcohol-associated cirrhosis with acute-on-chronic liver failure (ACLF), in the absence of SBP. Likewise we report a gut microbiota with extremely polarized and frank loss of bacterial diversity associated with a poor prognosis.
Case Report
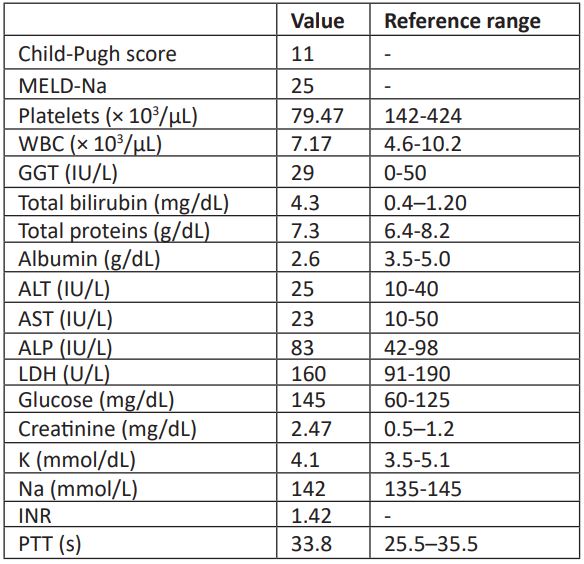
A 51-year-old Mexican male patient was admitted to the gastroenterology service of the Hospital Civil Fray Antonio Alcalde (Guadalajara, Mexico) due to altered mental status and spontaneous leakage of ascitic fluid through an umbilical hernia fistula. One week prior to hospital admission, the patient reported inversion of his sleep-wake cycle, in addition to progressive drowsiness and hallucinations. The patient was admitted with the following diagnoses: 1) Alcohol-associated cirrhosis, 2) ACLF grade II, precipitated by AKI II, and 3) type C HE (hepatic encephalopathy) West-Haven grade II. Table 1 shows clinical and laboratory evaluations at the time of hospitalization. After his admission, ammonia-lowering treatments (lactulose and rifaximin) and albumin were started, with poor response. Later, community-acquired pneumonia (CAP) was diagnosed and managed empirically with ceftriaxone and clarithromycin. Renal function worsened due to creatinine rising to 2.47 mg/dL and despite ammonia-lowering treatments, HE progressed to grade 4, which progressively worsened the respiratory pattern and management of respiratory secretions; despite this, the family refused orotracheal intubation. The patient presented cardiorespiratory arrest 48 hours after admission. Death was declared secondary to septic shock, severe CAP, ACLF grade 2, HE grade 4 and decompensated alcohol-associated cirrhosis.
Microbiology and molecular findings
Urine and peripheral blood cultures were obtained and were negative. 16S rRNA gene was amplified by PCR using broad-range primers and sequenced by Sanger method [3]. Moreover, blood and gut microbiota composition were assessed by bacterial and fungal metagenomic sequencing (V3-V4 regions of 16S rRNA and ITS2) [4].
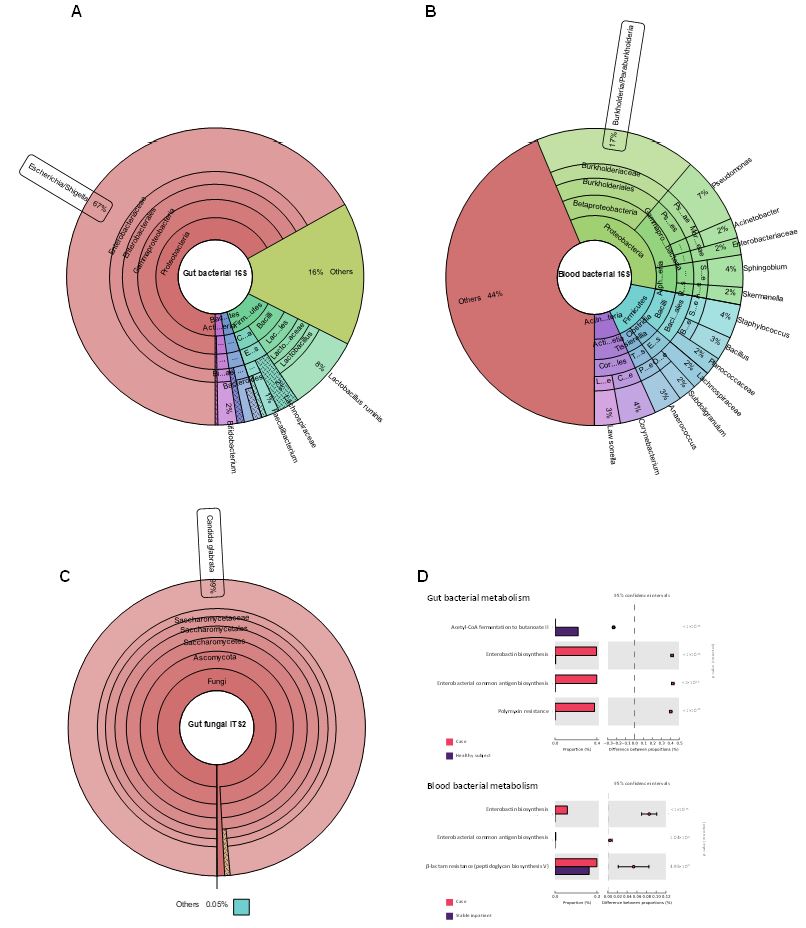
By means of broad-range 16S rRNA Sanger sequencing, we identified species of the Burkholderia cepacia complex at a 95% identity against sequences in the Gene Bank database in less than twenty four hours. Metagenomic sequencing of blood sample later re-confirmed the high abundance of Burkholderia genus (figure 1B). Metagenomic sequencing of the gut microbiota showed the dominance of the Proteobacteria phylum and a strong predominance of Escherichia/Shigella, comprising 67% of the gut microbiota (figure 1A). In contrast, this phylum represents only 4.5% in healthy subjects [5]. Predicted bacterial metabolism showed enhanced resistance to β-lactam and polymyxins, synthesis of enterobacterial antigens, and lack of butyrate production (figure 1D). Furthermore, fungal metagenomic sequencing revealed an absolute predominance of Candida glabrata (99% relative abundance, figure 1C).
Table 1: Blood test results and liver scores on admission.
Figure 1: Relative abundance of bacterial taxa in gut (A), blood (B) and fungal gut (C). Less abundant taxa are grouped as “others”. Plots were generated with Krona [6]. D) PICRUSt2 bacterial metabolism of the gut (upper) and blood (lower) microbiota of the patient compared with control subjects.
Discussion
Patients with cirrhosis and/or ACLF are characterized by pronounced gut dysbiosis, sustained bacterial translocation and an immunocompromised status. These factors predispose these patients to frequent bacterial and/or fungal infections with very high mortality [7]. At the moment of sample collection, the patient was under antibiotic therapy (ceftriaxone, clarithromycin and rifaximin). It has been described that empirical antibiotic therapy reduces the sensitivity of blood cultures in critical patients [8].
Umbilical hernia occurs in approximately 20% of patients with liver cirrhosis complicated with ascites [9]. Because of the enormous intra-abdominal pressure secondary to the ascites, umbilical hernia in these kinds of patients tends to enlarge rapidly and sometimes develop complications, such as Flood syndrome, or spontaneous umbilical hernia rupture. Several causes of rupture are increased intra-abdominal pressure associated with ascites and weakness of the abdominal wall muscles due to poor nutritional status. This rupture may follow a sudden increase in intra-abdominal pressure, such as vomiting, straining to defecate, or, in our patient's case coughing due to CAP. Complications of umbilical hernia rupture include hypotension secondary to large-volume spontaneous paracentesis, and the development of cellulitis, secondary peritonitis, and sepsis.
In our patient's case, we found neither any intestinal perforation nor data of secondary peritonitis. He had spontaneous drainage of ascitic fluid through his umbilical hernia fistula, without fever, vomiting, and no signs of peritoneal irritation, abdominal pain or abdominal tenderness. Unfortunately, analysis of the ascitic fluid was not performed due to absence of symptoms or signs of peritoneal irritation; but the patient received prophylactic antimicrobial therapy (ceftriaxone and clarithromycin) which could have coverage against of principal bacteria involved in bacterial peritonitis. Urine and peripheral blood cultures were negative and pneumonia was the unique infection cause found.
In the management of patients under empirical antibiotic therapy, which decrease the sensitivity of cultures, the use of molecular biology tools can serve as support in the detection and diagnosis of infections. In our patient, using this methodology, we were able to detect Bcc as a presumed infectious agent.
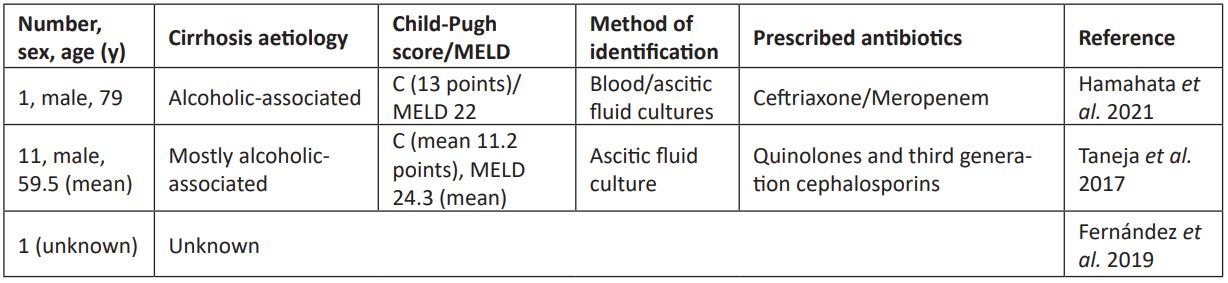
The Bcc is inherently resistant to a wide variety of antibiotics, including first and second-generation cephalosporins [10].. It usually causes infections in immunocompromised patients. This infection in cirrhotic patients are rare in the literature (table 2). In India, eleven cases of patients with cirrhosis and SBP associated with Bcc have been reported [2]. One case was reported in Japan, in which Bcc was detected systemically and associated with SBP [11]. In these cases, the infection was associated with the development of hepatorenal syndrome and the causes of death were septic shock and multi-organ failure. One more case was reported in Germany, however there is no specific operational definition for the case or infection site data [12].
Table 2: Cases of Bcc infection in liver cirrhosis patients.
Considering the metagenomic findings, the predominance of the Proteobacteria phylum in the gut (67%) and blood microbiota (34%), can be highly correlated. Given that Proteobacteria encompasses Bcc, and that sustained bacterial translocation is a hallmark of liver cirrhosis [7], evidence suggests that the probable origin of the infection could be due to bacterial translocation from the intestinal mucosa. In support of this hypothesis, there is evidence that Burkholderia can be found in the intestinal mucosa of patients with cirrhosis and HE [13]. Additionally, gut and blood microbiota also share antibiotic-resistance features related to Proteobacteria members, such as Escherichia/Shigella and Bcc, as evidenced by predicted bacterial metabolism. It is less probable that this infection was acquired from the hospital environment, and the existence of an in-hospital Bcc outbreak was ruled out.
Additionally, we found a profoundly dysbiotic gut fungal microbiota, dominated by C. glabrata (99%). Fungal microbiota has been barely described, and even less so in cirrhotic patients; therefore, no fungal microbiota profiles as polarized as in this case have been reported to date. It is to note that the stool and peripheral blood samples were collected thirty minutes before the patient's death, thus reflecting a particular microbiome profile just prior to the time of death.
The few reports on fungal microbiota in cirrhosis point that the relative abundance of Candida increases after the use of antibiotics for the treatment of HE or as prophylaxis for SBP, as in this case [14]. In cirrhotic patients, fungal infections often present as spontaneous fungal peritonitis (SFP) [15]. Notably, C. glabrata, which is associated with SFP, is also resistant to fluconazole [16]. Hence, we believe that monitoring fungal microbiota in cirrhotic patients should also be considered, especially since fungal dysbiosis polarized towards C. glabrata is a strong indicator of poor prognosis and that the rate of diagnosis of fungal infections in cirrhotic patients and their adequate treatment is notably low [15,17].
In summarize, we report the first clinical case of systemic Bcc infection, in a patient with decompensated cirrhosis complicated by ACLF II, without evidence of SBP and its blood and gut microbiota. Albeit gold-standard cultures were negative, Sanger sequencing was fast and effective to detect infection, which was later re-confirmed by metagenomic sequencing. Metagenomic sequencing also showed evidence that points bacterial translocation as a possible source of systemic infection. This case emphasizes the importance of implementing timely and accurate molecular diagnostic tests for both systemic and intestinal identification of pathogens in these patients, which are frequently resistant to antibiotics and probably optimize the antifungal usage.
Acknowledgement: We are grateful to Donovan Cortina-Romero for his help in collecting samples.
References
- Sfeir MM. Burkholderia cepacia complex infections: More complex than the bacterium name suggest. J Infect 2018; 77: 166-70.
- Taneja S, Kumar P, Gautam V et al. Spontaneous Bacterial Peritonitis by Burkholderia cepacia Complex: A Rare, Difficult to Treat Infection in Decompensated Cirrhotic Patients. J Clin Exp Hepatol 2017; 7: 102-6.
- Mishra D, Satpathy G, Chawla R et al. Utility of broad-range 16S rRNA PCR assay versus conventional methods for laboratory diagnosis of bacterial endophthalmitis in a tertiary care hospital. Br J Ophthalmol 2019; 103: 152-6.
- Baltazar-Díaz TA, González-Hernández LA, Aldana-Ledesma JM et al. Escherichia/Shigella, SCFAs, and Metabolic Pathways—The Triad That Orchestrates Intestinal Dysbiosis in Patients with Decompensated Alcoholic Cirrhosis from Western Mexico. Microorganisms. 2022; 10: 1231.
- Shin NR, Whon TW, Bae JW. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015; 33: 496-503.
- Ondov BD, Bergman NH, Phillippy AM. Interactive metagenomic visualization in a Web browser. BMC Bioinformatics. 2011; 385.
- Bruns T, Zimmermann HW, Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014; 20: 2542.
- Cheng MP, Stenstrom R, Paquette K et al. Blood Culture Results Before and After Antimicrobial Administration in Patients With Severe Manifestations of Sepsis: A Diagnostic Study. Ann Intern Med. 2019;171: 547-54.
- Coelho JC, Claus CM, Campos AC et al. Umbilical hernia in patients with liver cirrhosis: A surgical challenge. World J Gastrointest Surg. 2016; 8: 476.
- Rhodes KA, Schweizer HP. Antibiotic resistance in Burkholderia species. Drug Resist Updat. 2016; 28: 82-90.
- Hamahata A, Mitsusada S, Iwata T et al. Liver Cirrhosis Complicated by Spontaneous Bacterial Peritonitis Caused by the Burkholderia cepacia Complex. Intern Med. 2021; 60: 3435-40.
- Fernández J, Prado V, Trebicka J et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019; 70: 398-411.
- Bajaj JS, Hylemon PB, Ridlon JM et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol - Gastrointest Liver Physiol. 2012; 303: 675-85.
- Bajaj JS, Liu EJ, Kheradman R et al. Fungal dysbiosis in cirrhosis. Gut. 2018; 67: 1146-54.
- Fernández J, Acevedo J, Wiest R et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut. 2017; 67: 1870-80.
- Hwang SY, Yu SJ, Lee JH et al. Spontaneous fungal peritonitis: A severe complication in patients with advanced liver cirrhosis. Eur J Clin Microbiol Infect Dis. 2014; 33: 259-64.
- Yang AM, Inamine T, Hochrath K et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest 2017; 127: 2829-41.