Case report - Volume 3 - Issue 4
Hybrid intraoperative sutured stenting for anastomotic leak after total gastrectomy
Maria-Malvina Eleftheriou1; Τania Triantafyllou2; Kyriakos Bananis3; Dimosthenis Chrysikos 1,4; Dimitrios Theodorou1
1Department of Propaedeutic Surgery, Athens Medical School, National and Kapodistrian University of Athens, Hippocration General Hospital of Athens, Athens, Greece.
2UGI and General Surgery Department, NHS Lothian, Royal Infirmary of Edinburgh, Edinburgh, Scotland.
3General Surgery Department, NHS Trust, Ealing Hospital, London North West University Hospital, London, UK.
4Department of Anatomy, Medical School, National and Kapodistrian University of Athens, Greece
Received Date : June 14, 2023
Accepted Date : July 13, 2023
Published Date: July 20, 2023
Copyright:© Maria-Malvina Eleftheriou 2023
*Corresponding Author : Maria-Malvina Eleftheriou, Department of Propaedeutic Surgery, Athens Medical School, National and Kapodistrian University of Athens, Hippocration General Hospital of Athens, Athens, Greece. Tel : +00302132088142,
Email: malvina_el@hotmail.com
DOI: Doi.org/10.55920/2771-019X/1502
Abstract
Objective: The esophagojejunal anastomotic leak after total gastrectomy is a potentially fatal complication. Awareness, early diagnosis and innovations in interventional gastroenterology have significantly minimized the mortality rates. Endoscopic stenting has been previously introduced as an alternative therapeutic option. Migration of the stent, however, is a fearful complication.
Case report: We report the case of an 80-year old patient with anastomotic leak after total gastrectomy who was treated with intraoperative sutured stenting, which is our proposed modified technique.
Conclusion: The results of this approach seem favorable as the patient had a quick and uneventful recovery.
Keywords: Esophagojejunal anastomotic leak; total gastrectomy; intraoperative stenting; postoperative complications; gastric malignancy
Introduction
Total gastrectomy with Roux en Y reconstruction is the mainstay in the treatment of gastric cancer, with survival rates up to 98% for early stage gastric cancer [1-3]. Esophagojejunal anastomotic leak (EJAL) is a potentially fatal postoperative complication with an incidence reported up to 15% and mortality up to 60% [2,4-9]. EJAL may be attributed to patient- related or surgical technique- related factors. Management of the anastomotic defect and optimal supportive care of the patient can be challenging and requires a multimodal approach.
Evaluating the size of the defect and monitoring the output of the drains are essential steps towards designing the treatment options [9-11]. Non-operative treatment is preferred for small leaks and imaging-guided percutaneous drainage of abscesses or effusions may be adequate. Supportive therapy with bowel rest, parenteral nutrition and antibiotics is also substantial [4-6,9]. Endoscopic treatment aims to seal the defect and consists of stents, clips, endoscopic suturing, glue or fibrin sheets [2,11]. The surgical approach is reserved for patients with uncontrolled sepsis or failure of the conservative or endoscopic measurements.
Endoscopic stenting is a quick and well-tolerated procedure [12]. After successful stenting, oral diet can be resumed. The overall success rate has been reported between 62-90% [2,8- 9,12]. However, the complication rate is estimated approximately 60% including migration of the stent [13-14]. Small bowel obstruction has been reported in 8% of the interventions [14]. 20% of the patients present mucosal friability due to chronic pressure of the stent towards the mucosa, 10% tissue growth and 1-10% bleeding after removal [13-14]. We present the case of an 80-year-old patient diagnosed with EJAL who was successfully treated with endoscopic stenting.
Case presentation
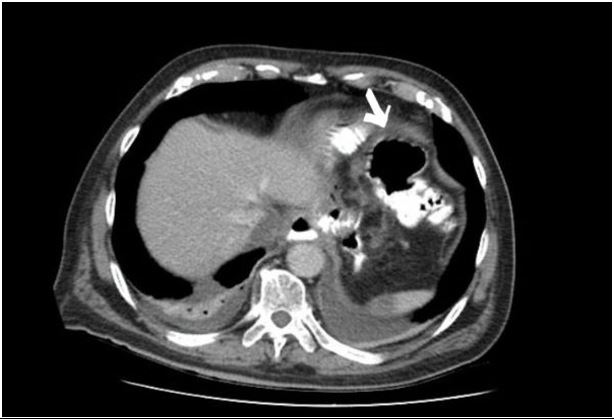
An 80-year-old male patient with a diagnosis of gastric adenocarcinoma underwent elective total gastrectomy with Roux-en-Y reconstruction in our specialized unit. An abdominal drain was placed subhepatically. He presented persistent leukocytosis since the first postoperative day. During the fifth postoperative day, the patient developed abdominal pain and fever. Computer Tomography with water-soluble oral contrast revealed an anastomotic esophagojejunal leak along with a 4 x 4 cm abscess in the gastrosplenic space (Fig.1).
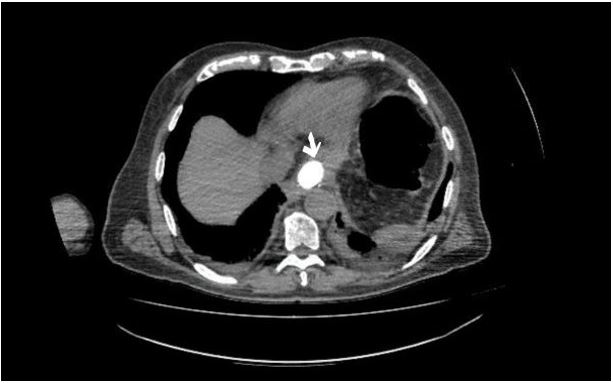
After initiating intravenous fluids and antibiotics, an exploratory laparotomy was performed. Drainage of the abscess and thorough wash-out of the abdominal cavity were completed. Intraoperative endoscopic stenting was decided. After identifying a 2 cm defect in the site of the anastomosis endoscopically, a 10cm fully covered self-expanding metal stent (SEMS) (Evolution Esophageal Controlled-Release Stent–Cook Medical, USA) was advanced through the anastomosis. The stent was then fixed through the abdominal incision with a 3-0 Vicryl stitch. The drain tube was repositioned in place. Plain radiography series confirmed the proper location of the stent postoperatively (Fig.2). The patient was discharged seven days after the reoperation on puree diet having the stent removed six weeks after the reoperation.
Figure 1: Computed Tomography findings of the EJAL. The arrow shows towards the 4x4 abscess.
Figure 2: Computed Tomography findings after the re-operation. The arrow shows towards the oral contrast-full stent.
Discussion
The EJAL is one of the most fearful surgical complications. Age over 65, male sex, cardiopulmonary comorbidities and diffuse histology of the tumor have been reported as risk factors. Extended lymphadenectomy, intraoperative blood loss, anastomotic tension and compromised blood supply have also been discussed [4, 6-7, 15]. Nutritional support, reinforcement of anastomosis with fibrin or tissue patches, reconstruction of the anastomosis have all been proposed as therapeutic options. Early diagnosis and management of an EJAL are key in avoiding sepsis and prevent further clinical deterioration.
Endoscopic stenting is considered one of the most successful approaches for postoperative leaks after foregut surgery [8, 10, 12-13]. However, stent migration is a severe complication. Researchers have attempted stent fixation with endoscopic clips [4, 8- 10]. Transnasal silk thread has also been used [2, 4, 12]. Over-the-scope clips, fibrin glue with or without supplementary polyglycolic acid sheets, vacuum therapy, endoscopic stitching and placement of endoscopic drains require optimal collaboration between the gastroenterologists, the surgical and anaesthetic teams [2,4,8,11-12].
In the case presented, the combined endoscopic and surgical approach was designed for the EJAL. Instead of reconstructing the anastomosis, a SEMS was advanced intraoperatively. The stent’s position was secured using a 3-0 Vicryl suture, which is fully absorbed within 56 to 70 days [2,12].
To date, only a few reports of sutured stenting for EJAL after total gastrectomy have been published. The technique has also been applied in patients with leak after bariatric procedures [12]. Bridging the defect and draining the peritoneal collections or abscesses is a quick and safe operation that seals the leak and allows for adequate healing. Whether the stent interferes with the already compromised microcirculation of the anastomosis remains debatable.
Conclusion
Intraoperative endoscopic stenting can be a safe alternative for patients who are candidates for reoperation and whose potential reconstruction of the anastomosis may result in major postoperative morbidity. Stenting anastomotic defects and securing the stent with intraoperative suturing may be the key in avoiding migration and further complications while allowing for adequate healing of the EJAL.
References
- Sweigert PJ, Eguia E, Nelson M, Nassoiy S, Knab L, Abood G, et al. Total gastrectomy in patients with gastric adenocarcinoma: Is there an advantage to the minimally invasive approach? Surgery. 2019; 166(4): 623-31.
- Choi C, Kang DH, Kim HW, Park SB, Kim SJ, Hwang SH, et al. Full covered self-expandable metal stents for the treatment of anastomotic leak using a silk thread. Medicine (Baltimore). 2017; 96(29): e7439.
- Saragoni L, Scarpi E, Ravaioli A, Morgagni P, Roviello F, Vindigni C, et al. Early Gastric Cancer: Clinical Behavior and Treatment Options. Results of an Italian Multicenter Study on Behalf of the Italian Gastric Cancer Research Group (GIRCG). Oncologist. 2018; 23(7): 852-58.
- Gong W, Li J. Combat with esophagojejunal anastomotic leakage after total gastrectomy for gastric cancer: A critical review of the literature. Int J Surg. 2017 Nov; 47:18-24.
- Hajjar NA, Popa C, Al-Momani T, Margarit S, Graur F, Tantau M. Esophagojejunal anastomosis fistula, distal esophageal stenosis, and metallic stent migration after total gastrectomy. Case Rep Surg. 2015; 2015: 839057
- Trapani R, Rausei S, Reddavid R, Degiuli M, Italian Research Group for Gastric Cancer (GIRCG) Clinical Investigators. Risk factors for esophago-jejunal anastomosis leakage after total gastrectomy for cancer. A multicenter retrospective study of the Italian research group for gastric cancer. Eur J Surg Oncol. 2020;4 6(12): 2243-7.
- Xing J, Liu M, Qi X, Yu J, Fan Y, Xu K, et al. Risk factors for esophagojejunal anastomotic leakage after curative total gastrectomy combined with D2 lymph node dissection for gastric cancer. J Int Med Res. 2021; 49(3):3000605211000883.
- Hoeppner J, Kulemann B, Seifert G, Marjanovic G, Fischer A, Hopt UT, et al. Covered self-expanding stent treatment for anastomotic leakage: outcomes in esophagogastric and esophagojejunal anastomoses. Surg Endosc. 2014; 28(5): 1703-11. 10.1007/s00464-013-3379-4
- Aurello P, Magistri P, D'Angelo F, Valabrega S, Sirimarco D, Tierno S, et al. Treatment of esophagojejunal anastomosis leakage: a systematic review from the last two decades. Am Surg. 2015; 81(5): 450-3.
- Miłek T, Ciostek P, Petryka R, Słowik J, Jarosz M. Results of endoscopic and surgical fistula treatment in oesophagointestinal anastomosis after gastrectomy. Wideochir Inne Tech Maloinwazyjne. 2016; 10(4): 515-20.
- Choi SI, Park JY. Large anastomotic leak: endoscopic treatment using combined fibrin glue and polyglycolic acid (PGA) sheets. BMJ Case Rep. 2021; 14(8): e240188.
- Raimondo D, Sinagra E, Facella T, Rossi F, Messina M, Spada M, et al. Self-expandable metal stent placement for closure of a leak after total gastrectomy for gastric cancer: report on three cases and review of the literature. Case Rep Gastrointest Med. 2014; 2014: 409283.
- Aryaie A, Singer J, Fayezizadeh M, Lash J, Marks JM. Efficacy of endoscopic management of leak after foregut surgery with endoscopic covered self-expanding metal stents (SEMS). Surg Endosc. 2017; 31(2): 612-7.
- Singer JL, Aryaie AH, Fayezizadeh M, Lash J, Marks JM. Predictive Factors for the Migration of Endoscopic Self-Expanding Metal Stents Placed in the Foregut. Surg Innov. 2017; 24(4): 353-7.
- Rodríguez-Quintero JH, Aguilar-Frasco J, Morales-Maza J, Sánchez-García-Ramos E, Medina-Franco H, Cortes-Gonzalez R. Predictors of anastomotic leak after total gastrectomy in patients with adenocarcinoma. Cir Cir. 2022; 90(2): 216-22.