Research Article - Volume 3 - Issue 4
The use of clinical, laboratory, and radiologic data in the assessment of pediatric extrapulmonary tuberculosis cases in Kisumu County, Kenya
Silas O. Awuor1*; Eric O. Omwenga2; Richard M. Mariita3; Hellen Ogollah4; Timothy Malika5; Hezrone Okoth1; Tobias Nyanjong1; Grace Ndeda1
1Jaramogi Oginga Odinga Teaching and Referral Hospital, P.O Box 849, Kisumu, Kenya.
2Kisii University, School of Health Sciences, P.O. Box 408 - 40200, Kisii, Kenya.
3Microbial BioSolutions, Troy, New York, 12180 USA.
4Ministry of Health, County laboratory coordinator- Kisumu County, Kenya.
5Ministry of health, County TB coordinator- Kisumu County, Kenya.
Received Date : June 19, 2023
Accepted Date : July 18, 2023
Published Date: July 25, 2023
Copyright:© Jessica Kern 2023
*Corresponding Author : Silas O. Awuor, Jaramogi Oginga Odinga Teaching and Referral Hospital, P.O Box 849, Kisumu, Kenya.
Email: awuorhsd@otti.ac.ke
DOI: Doi.org/10.55920/2771-019X/1507
Abstract
Background: Extrapulmonary tuberculosis is detected more frequently in children than adults since the risk of lymphohematogen spread is high in young children. Tuberculosis may attack any organ in the body, including the lymph nodes and the central nervous system. In children, it is difficult to detect tuberculosis since most signs and symptoms are not easily detected in young children. However, it has been documented to be the most crucial cause of mortality in the childhood age group. It has also been found to be non-specific, with low sensitivity to diagnostic tests amongst pediatric patients. This is partly because of its ability to mimic many other disease manifestations. This study evaluated the clinical, laboratory, and radiologic observations and treatment outcomes in pediatric patients followed up in the TB clinic.
Material and Methods: All the pediatric patients who were aged 0 - 216 months attending TB-clinic at Jaramogi Odinga Oginga Teaching and Referral Hospital as from June 2021 to June 2022 and their parent sign the consent were included in this study giving a sample size of eighty patients.
Results: Eighty patients, 50 (62.5%) were females, in which 29 (36.3%) patients were aged 0–48 months, 20 (25%) were aged 60–108 months, and 31 (38.8%) patients were aged 120–216 Months. Out of 80, 50 (62.5%) patients were diagnosed with extrapulmonary tuberculosis, while 30 (37.5%) had pulmonary and extrapulmonary tuberculosis. Common form of extrapulmonary tuberculosis was extrathoracic lymphadenopathy with 26 (32.5%) patients, followed by musculoskeletal system tuberculosis 14 (17.5%), gastrointestinal system tuberculosis 10 (12.5%), miliary tuberculosis, and intrathoracic lymphadenopathy both at 8 (10%), renal tuberculosis 6 (7.5%), central nervous system tuberculosis 5 (6.3%) and lastly pleural tuberculosis 3(3.8%). The median treatment period was 12 (range, 6–24) months.
Conclusion: Clinical, laboratory, and radiologic data should be evaluated together when diagnosing extrapulmonary tuberculosis in children. Interferon-gamma release tests alone are not superior to the tuberculin skin test but should be used in combination during the diagnosis.
Keywords: Child, extrapulmonary tuberculosis, evaluation
Introduction
Mycobacterium tuberculosis belonging to Genus Mycobacterium is the predominant species causing Tuberculosis (TB), which primarily affects the lungs. It mainly spread through aerosols when the infected cough, sneeze or spit is inhale by an individual. According to the World Health Organization (WHO) 2017 report, the mortality rate has reduced by 37% but still remains top killer due to its antibiotic resistance pattern and co-infection with HIV [1]. Ten million people get sick from TB annually, with 1.5 million people succumbing to TB each year, making it the world’s top infectious killer. World Health Organization (WHO) 2021 reported that children aged below 15 years constituted approximately 12% of 10.4 million new tuberculosis cases in 2020. In the same report, it was documented that 66% of 12,417 new cases reported in Kenya were pulmonary tuberculosis.
Extrapulmonary tuberculosis (EP-TBC) is seen more in children compared to adults since the threat of lymphohematogen spread is high in young children which has attributed to the fact that Tuberculosis may affect any organ in the body including lymph nodes and the central nervous system (CNS) [2]. Attention should be given to extrapulmonary organ involvement in patients diagnosed with pulmonary tuberculosis as treatment will be administered for prolonged period, especially in CNS tuberculosis, and bone and joint tuberculosis. It is hard to diagnose tuberculosis in children since most signs and symptoms of tuberculosis are non-specific, and the sensitivity to diagnostic tests are low in pediatric patients, with the fact that tuberculosis infection may mimic many other disease entities [3]. However, most substantial factor that marks morbidity and mortality rates is the early initiation of treatment as it enhances its spread [4]. Therefore, it is advisable that treatment should be started after assessing clinical and radiologic findings together when it is impossible to verify the disease through laboratory findings [4]. In this regard, the publication of tuberculosis data in children in our country is considered vital as much has not been documented. In this article, we aimed to contribute to our country’s data by assessing the clinical, laboratory, and radiologic findings and treatment results of our pediatric patients who were followed up with a diagnosis of EP-TBC in JOOTRH TB-clinic as from June 2021 to June 2022.
Material and Methods
Study site
This study was carried at the Jaramogi Oginga Odinga Teaching & Referral Hospital, which is the only referral hospital in the county and it serve the counties bordering it, the hospital is located along Kisumu Kakamega Road.
Sample size
All the pediatric patients who were aged 0 - 216 months attending TB-clinic at Jaramogi Odinga Oginga Teaching and Referral Hospital as from June 2021 to June 2022 and their parent sign the consent were included in this study. A total of Eighty pediatric patients of the age group was achieved and included in the study. The sex, age, history of contact with tuberculosis, symptoms at the time of presentation, physical examination findings, laboratory, radiologic, and microbiologic data, and treatment regimens belonging to these patients were examined from the patient files and laboratory register.
Clinical evaluation
The evaluation was performed by obtaining clinical examinations of the most common symptom such as neck swelling, fever and night sweating, restricted movement and pain in the extremities, hematuria, cough, weight loss, blurred consciousness, vomiting, headache growth, and developmental delay which was achieved from the patient of interest records through the assistance of the clinician working in the department.
Radiologic evaluation
The radiologic imaging of all patients at baseline and the end of treatment were evaluated using posteroanterior lung radiography and a thoracic computed tomography scanner. Patients with EP-TBC were screened in terms of tuberculosis-specific radiologic findings by infection site.
Microbiologic evaluation
The assessment was based on previously used protocols as per CLSI guidelines [15]. Briefly, sputum, fasting gastric juice, bronchoalveolar lavage fluid, tuberculin skin test, interferon-gamma release test, and CSF samples obtained through a lumbar puncture in appropriate cases and tissue samples obtained by the organ involved were used for microbiologic tests. All these samples were obtained by use of standard operating procedures [15]. In addition, direct smears, Auramine O was explored and GeneXpert performed.
Statistical analysis
The Statistical Package for the Social Science (SPSS) (Version 21, Chicago) statistical program was utilized for statistical analyses. Qualitative measurements were expressed as numbers and percentages while quantitative measurements were expressed as mean ± standard deviation (by specifying median, minimum and maximum values, when necessary).
Ethical consideration
As per ethical guidelines, confidentiality and privacy were strictly adhered to, and no names of individuals were recorded or made known in the collection or reporting of information. Approval to conduct the study was sought from the Institutional Research Ethics Committee (IREC) at Jaramogi Oginga Odinga Teaching and Referral Hospital (JOOTRH).
Results
Demographic data and clinical evaluation findings
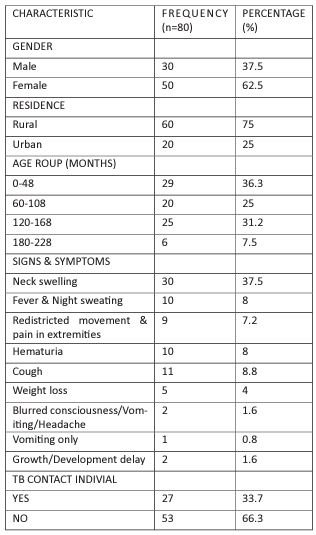
Of the 80 patients, 50 (62.5%) were female, and 30 (37.5%) were male, with higher number of patients (60 (75%)), coming from the rural area. The median age of the patients was 106 (range, 5–204) months. Twenty-nine patients (36.3%) were aged between 0 - 48 months, 20 (25%) were aged between 60-108 months, and 31 (38.8%) were aged between 120-216 months. On clinical findings and laboratory examinations, the most common symptom at presentation was the presence of neck swelling, which was found in 30 (37.5%) patients. Fever and night sweating were present in 10 patients (8%), restricted movement and pain in the extremities were present in 9 (7.2%), the cough was present in 11 (8.8%), hematuria was present in 10 (8%), and weight loss was present in 5 (4%) patients. Blurred consciousness, vomiting, and headache were found in 2 (1.6%), vomiting was found in 1 (0.8%) patients, and growth and developmental delay was found in 2 patients (1.6%). As a result of history and family screening, contact with an individual with tuberculosis in the immediate vicinity was found in 27 (33.7%) patients, as shown in Table 1
Table 1: Demographic and clinical evaluation findings.
Microscopic evaluation
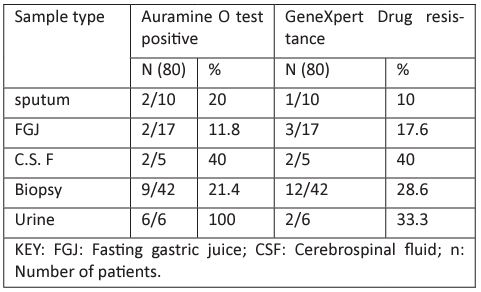
Diagnostic evaluation Auramine O positivity was found as 21 (16.8%) in various body fluids and tissue samples. On GeneXpert, the positivity rate was 20 (16%), as shown in Table 2 in which5(4%) patients had rifampicin (R) resistance.
Table 2: Microscopic data belonging to the cases of tuberculosis diagnosed at TB clinic at JOOTRH.
(16.8%) in various body fluids and tissue samples. On GeneXpert, the positivity rate was 20 (16%), as shown in Table 2 in which5(4%) patients had rifampicin (R) resistance.
Radiographic evaluation findings and the TB treatment
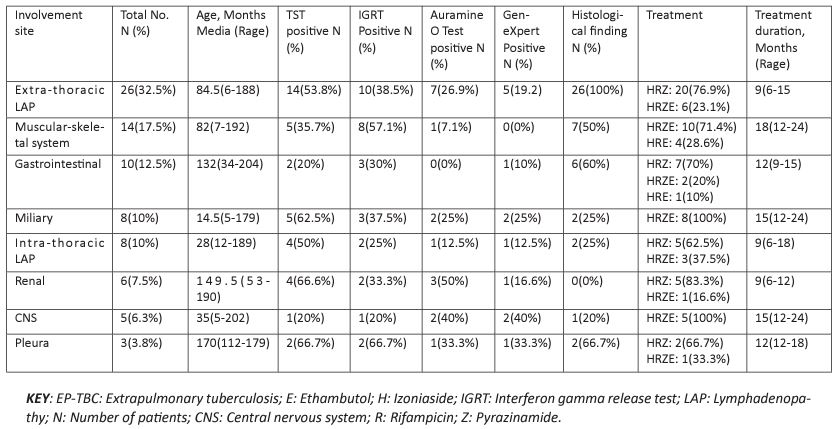
Fifty (62.5%) patients revealed EP-TBC, and 30 (37.5%) had pulmonary TBC+EP-TBC. On the distribution of the extrapulmonary tuberculosis, it was found that 26 (32.5%) had extra-thoracic lymphadenopathy (LAP) followed by musculoskeletal system tuberculosis 14(17.5%), gastrointestinal system tuberculosis 10(12.5%), miliary tuberculosis and intrathoracic lymphadenopathy both at 8 (10%), renal tuberculosis 6 (7.5%), central nervous system tuberculosis 5 (6.3%) and lastly pleural tuberculosis 3 (3.8%). The median treatment period was 12 (range of 6–24) months. The most used treatment regimen was HR+pyrazinamide (Z) combination, which was used in 38 (30.4%) patients, Ethambutol (E)+HRZ was used in 36(28.8%) patients. HRE was utilized in 5 (4%) patients and HRS+para-aminosalicylic acid (PAS) combination was used in 1 (0.8%) patient as in Table 3.
Table 3: Distributions of the patients on TB care management at JOOTRH TB clinic from June 2021 to June 22.
Discussion
From the study, extrapulmonary organ involvement was found to be advanced, with a rate of 62.5% while 37.5% had pulmonary + extrapulmonary tuberculosis; it was noted that these rates are more significant in the age group below 120 months. In this age group, the occurrence of EP-TBC increases because the risk of lymphohematogenous dissemination is high [5]. Equally, in our study, children aged between 0 and 48 months instituted more than one-third of our patients diagnosed with EP-TBC. In contrast to adults, the possibility of accompaniment of EPTBC in pulmonary tuberculosis is increased in childhood. From this finding, it should be emphasized that a high level of notion should be maintained in terms of screening extrapulmonary organ involvement when a diagnosis of pulmonary tuberculosis is made, especially in young children. Tuberculosis lymphadenitis is generally the most common form of EP-TBC [6], and from this study, the most common form of EP-TBC was extrathoracic lymphadenopathy (32.5%). This study shows similarity to the study conducted by Coşar et al. [7] in which childhood tuberculosis was evaluated and the frequency of EP-TBC was found as 38.6%, and the frequency of tuberculosis lymphadenitis was found as 11.7%.
According to the statistics of our country, pleural tuberculosis is the second most common extrapulmonary tuberculosis, including children aged below 1180 months. Bone-joint involvement is rarely observed and constitutes 3% of all tuberculosis cases [6]. Its frequency among extrapulmonary tuberculosis cases has been reported to be about 10–35%. In our case series, the second most common extrapulmonary tuberculosis was found to be musculoskeletal tuberculosis (17.5%) in disparity to country-wide data. Pleural tuberculosis alone was the oddest form of EP-TBC. This may be related to the fact that complex cases were referred to us because our hospital is the referral hospital within the county.
The most severe form of extrapulmonary tuberculosis is miliary tuberculosis and CNS tuberculosis. It has been reported that the risk of development of these two morbidities is high, especially in children aged between 6 months and 48 months [8-11]. Central nervous system tuberculosis may be observed as parenchymal or meningeal tuberculosis [12]. Therefore, investigation in terms of miliary tuberculosis and possible CNS tuberculosis, especially in children aged below 4 years will reduce the rates of complications and mortality
Statistics related to the complications of tuberculosis meningitis in children show variance in the literature. In this study, the median age was found as 96, 96 (range, 0, 48–204) months in the patients who had CNS tuberculosis, one patient died of herniation during the follow-up period and ventriculoperitoneal shunts were applied to the patient. In agreement with the literature data, TST and IGRT positivity rates were found to be low in our patients who had CNS tuberculosis. However, TSTs were found to be positive with a reasonably high rate (62.5%) in our patients who had miliary tuberculosis. This finding shows some similarity with other study done before, like in another article reported from Vietnam, the mortality rate was reported as 15% and the rate of neurologic sequala was reported as 33% in children with tuberculosis meningitis (13). While in the study done by Anjum et al. [20] in Pakistan, the mortality rate was found as 5% in 40 children with tuberculosis meningitis, and it was reported that neurologic sequela developed in all patients who survived.
Treatment of extrapulmonary tuberculosis is generally similar to that of pulmonary tuberculosis, but its duration may be longer according to the section involved. However, it can be stated that children generally tolerate anti-tuberculosis drugs better than adults [14]. In this study, elevated transaminase levels were found at a rate of 3.8% and anaphylaxis was found with a rate of 2.4%. In addition, the development of complications should be closely monitored in these patients who have high life expectancy because the possibility of extension of the disease is high. In our patient group, complications, including hydrocephalus, kyphoscoliosis, and hydronephrosis were observed in eight patients.
Conclusion
In conclusion, clinical, laboratory, and radiologic data should be evaluated in combination when diagnosing of EP-TBC in children. Interferon-gamma release tests alone are not superior to TST and they should be combined during diagnosis.
Competing interests
The authors declare no competing interests.
Authors’ contributions
All authors contributed equally to this work.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
All the data has been shared in the manuscript
References
- World Health Organization. Global tuberculosis report 2017. Geneva: World Health Organization; 2017.
- World Health Organization. WHO Tuberculosis Programme: Framework for Effective Tuberculosis. Control, 1994.
- Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med. 2012; 367: 348-61.
- Stop TB Partnership Childhood TB Subgroup World Health Organization. Guidance for National Tuberculosis Programmes on the management of tuberculosis in children. Chapter 1: introduction and diagnosis of tuberculosis in children. Int J Tuberc Lung Dis. 2006; 10: 1091-7
- Gündeşlioğlu ÖÖ, Kocabaş E. Extrapulmonary Tuberculosis in Childhood. Turkiye Klinikleri J Pediatr Sci. 2016; 12: 32-8.
- Bozdemir ŞE, Nazlıoğlu HÖ, Hacımustafaoğlu M, Çelebi S. Tuberculous Lymphadenitis in Children. J Pediatr Inf. 2012; 6: 6-11.
- Coşar H, Onay H, Bayram N, Özkınay F. The Evaluation of the Epidemiological and Clinical Findings and the Prognosis of the 44 Pediatric Tuberculosis Patients. J Pediatr Inf. 2008; 2: 1-6.
- Starke JR. Mycobacterium tuberculosis. In: Long SS, Pickering LK, Prober CG, editors. Principles and Practice of Pediatric Infectious Diseases. 4th ed. Philadelphia. 2012: 771-86.
- Gupta RK, Kumar S. Central nervous system tuberculosis. Neuroimaging Clin N Am 2011; 21: 795-814.
- CDC. Reported tuberculosis in the United States, 2004. Atlanta, GA: US Department of Health and Human Services, CDC. 2005.
- Sharma SK, Mohan A, Sharma A, Mitra DK. Miliary tuberculosis: new insights into an old disease. Lancet Infect Di.s 2005; 5: 415-30.
- Patkar D, Narang J, Yanamandala R, Lawande M, Shah GV. Central nervous system tuberculosis: pathophysiology and imaging findings. Neuroimaging Clin N Am. 2012; 22: 677-705.
- Bang ND, Caws M, Truc TT, et al. Clinical presentations, diagnosis, mortality and prognostic markers of tuberculous meningitis in Vietnamese children: a prospective descriptive study. BMC Infect Dis. 2016; 16: 573.
- Furin JJ, Mitnick CD, Shin SS, et al. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2001; 5: 648-55.
- Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement. 32nd Edition, M100 Clinical Laboratory Standards Institute. 2022.