Research article - Volume 3 - Issue 4
Schistosoma Mansoni infection and hepatocellular carcinoma: A co-morbidity study
Amal Farahat Allam*; Hoda Fahmy Farag; Amel Youssef Shehab; Ahmed Soliman El Sahy; Safia Saleh Khalil; Naglaa Fathi Abd El-Latifa
Department of Parasitology, Medical Research Institute, University of Alexandria, 165 El Horreya avenue, El Hadara, Alexandria, Egypt.
Received Date : June 30, 2023
Accepted Date : July 24, 2023
Published Date: July 31, 2023
Copyright:© Amal Farahat Allam 2023
*Corresponding Author : Amal Farahat Allam, Department of Parasitology, Medical Research Institute; Alexandria University, postal address; Medical Research Institute, 165 El Horreya avenue, El Hadara, Alexandria, Egypt.
Email: amalalam2005@yahoo.com
DOI: Doi.org/10.55920/2771-019X/1515
Abstract
Background: The implication of human Schistosoma mansoni (S. mansoni) infection in concomitance with other risk factors such as hepatitis C virus (HCV) and hepatitis B virus (HBV) in the development of hepatocellular carcinoma (HCC) is still under controversy. This work aimed
to evaluate the role of Schistosoma mansoni infection in association with hepatitis B virus (HBV) and hepatitis C virus (HCV) and other risk factors in the development and/or progress of HCC.
Methods: The present study was carried out on 90 HCC patients recruited from Kafr El-Sheikh Liver Disease Research Institute. After obtaining their informed consents, socio-demographic and clinical data were collected and patients were examined for S. mansoni by Kato-Katz and indirect hemagglutination (IHA) techniques. Alpha-fetoprotein (AFP) level was determined. The Child-Pugh scoring system and Barcelona Clinic Liver Cancer (BCLC) staging system were used to evaluate the pathological features of the studied patients.
Results: All participants were negative for active S. mansoni by Kato-Katz. Based on IHA, the participants were categorized into two groups: group I: sixty-two patients negative for S. mansoni and group II: twenty-eight positives. The patients’ age ranged between 40->60 years with a mean of 57.07± 8.12 years. HCC was more prevalent in the age range of >50-60 years in both groups. Males were more than females and rural participants were more than urban patients in both groups. Most of the patients had HCV (88.9%) while 7.8% had HBV. A higher proportion of HCC patients showed concomitant HCV and S. mansoni (92.6%) than the S. mansoni negative group. Alpha-fetoprotein (AFP) level was higher in group II than that in group I with no significant difference. Statistical analysis showed no difference between the two studied groups regarding Child scores. On the contrary, BCLC class D was significantly higher among HCC positive schistosomiasis cases compared to the negative group.
Conclusion: Concomitant S. mansoni with HCV and HBV potentiate HCC progression.
Keywords: Hepatocellular carcinoma, S. mansoni, HCV, HBV, Alpha-fetoprotein
Introduction
Schistosomiasis is a chronic disease caused by the genus Schistosoma of the trematode parasites. It is endemic in 78 countries, infecting more than 207 million people worldwide and provoking above 200,000 deaths yearly [1]. Human infection occurs after exposure to contaminated water with schistosome cercariae which penetrate the skin and become schistosomula. They penetrate the wall of a nearby vein and are carried out in the blood circulation, eventually reaching the portal venous system, where they mature and lay eggs. These eggs are either trapped in the tissues and provoke a granulomatous reaction or are passed in the feces, then hatch in water to miracidia which invade the snail and are developed into cercariae to start a new cycle causing schistosomiasis which affects several organs, including the genitourinary, digestive, and nervous systems causing a spectrum of serious diseases [2]. Different Schistosoma species are involved in the development and/or progression of some tumor types, including colorectal carcinoma, prostate adenocarcinoma, giant follicular lymphoma, and hepatocellular carcinoma (HCC) [1].
Hepatocellular carcinoma (HCC) is the sixth most widespread cancer in the world and is responsible for approximately one million deaths per year. Most of HCC develops in the presence of chronic liver diseases mostly related to hepatitis B virus (HBV) and hepatitis C virus (HCV) infections in addition to intestinal schistosomiasis [3-5].
Egypt ranks the 15th worldwide and the 3rd country in Africa regarding HCC. HCC constitutes 70% of all liver tumors, with an increase in the incidence rate in the last years [5-7]. This requires the investigation of the potential risk factors for its development. In rural areas, S. mansoni remained a major chronic disease for many years [8]. It develops a series of pathology including hepatic fibrosis and it can help in development of neoplasia due to chronic granulomatous inflammatory reactions [9]. There is controversy and limited evidence in the available literature about the real role of S. mansoni alone or in association with hepatitis B virus (HBV) and hepatitis C virus (HCV) in the development of hepatic carcinoma. It was reported that 70-90% of patients with chronic hepatitis, cirrhosis or HCC have co-infection of schistosomiasis and HCV [5, 10]. It was suggested that the combination of chronic S. mansoni infection and HBV or HCV may cause a higher risk of HCC. Understanding these associations may clarify the development and progress of HCC and subsequently draw attention and more effort to its prevention and control [7, 11-13].
Screening tools of HCC were commonly performed by measuring AFP levels in combination with ultrasonography that increased its sensitivity to 100% [14]. It is a large serum glycoprotein, belonging to the eccentric class of onco-development protein [15]. Normally, its level decreases sharply after birth and continues at a low level afterwards. AFP level was studied among HCC patients in combination with other factors to find out if it is correlated with the progress of the disease [16].
The severity of HCC is evaluated using the modified Child-Pugh scoring system and Barcelona Clinic Liver Cancer (BCLC) staging system [17, 18]. Child Pugh-score relies on clinical and laboratory evaluation including liver functions, serum albumin, international normalization ratio (INR) and prothrombin time to assess the severity of liver cirrhosis. BCLC identifies the stages of HCC based on the number and the size of tumors in the liver associated with liver function and overall performance status [19]. It helps in decision of treatment for HCC patients [20, 21].
To shed more light on this controversial issue, this work aimed to evaluate the role of Schistosoma mansoni infection in association with HBV and HCV and other risk factors in the development and/or progress of HCC.
Materials and Methods
Study Subjects and Ethical Considerations
To perform this study, approvals from the Egyptian Ministry of Health and Population, the administration of Health Affairs in Kafr El-Sheikh governorate and the Research Ethics Committee of the Medical Research Institute (MRI), Alexandria University were obtained. The study was conducted on 90 HCC patients from Kafr El Sheikh and nearby Gharbia governorates attending or admitted to Kafr El Sheikh Liver Disease Research Institute during the period from September 2018 to September 2019. They were diagnosed by ultrasound (US) and confirmed by Triphasic Computed Tomography (CT).
All participants were asked to freely volunteer and informed consent was obtained prior to their inclusion in the study. Based on a predesigned questionnaire, brief demographic and retrospective clinical signs were collected including age, gender, residency and medical records concerning upper gastrointestinal bleeding (GIB).
Laboratory Investigations
Stool and blood (2ml/patient) samples were collected from all patients and examined for active S. mansoni infection by Kato-Katz (Katz et al., 1972) [22] and IHA (Bilharziose Fumouze Diagnostics/SERFIB, France). Accordingly, the patients were categorized into two groups; group I included HCC patients negative for schistosomiasis and group II included HCC patients with schistosomiasis. They were also examined for HCV antibodies by enzyme-linked immunosorbent assay (ELISA) technique (Human diagnostics, Germany and HCV Murex 40 Anhet laboratories, USA).
The results of HBsAg and the other routine laboratory tests that were processed for each HCC patient were collected from the institute’s records. Among these tests: liver enzymes, alanine aminotransferase (ALT/GPT), aspartate aminotransferase (AST/GOT), bilirubin (total and direct), albumin, thromboplastin activity and international normalization ratio (INR) in plasma using Biomed-Liquiplastin kit. No patients had concomitant parasite infections and any serological evidence, or a history of recent or old hepatitis A or D virus infections. Serum AFP level was performed for each patient using ELISA technique (AFP ElISA kit UK)[23, 24].
Evaluation of HCC Severity
The severity of liver cirrhosis was evaluated in each patient with a modified Child-Pugh score [17]. The BCLC stages were determined based on tumor features and liver function tests [18]. Accordingly, patients were classified as A (early), B (intermediate), C (advanced) and D (terminal) [12]. All the data were obtained from medical records.
Statistical Analysis
Data analysis was completed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp) [25]. Qualitative data was described using numbers and percentages. The Kolmogorov-Smirnov test was used to verify the normality of distribution of quantitative data using range (minimum and maximum), mean and standard deviation, median and interquartile range (IQR). The significance of the obtained results was judged at the 5% level. Tests of Chi-square, Fisher’s Exact, Mann Whitney and student t-test were used to compare between the two studied groups [26].
Results
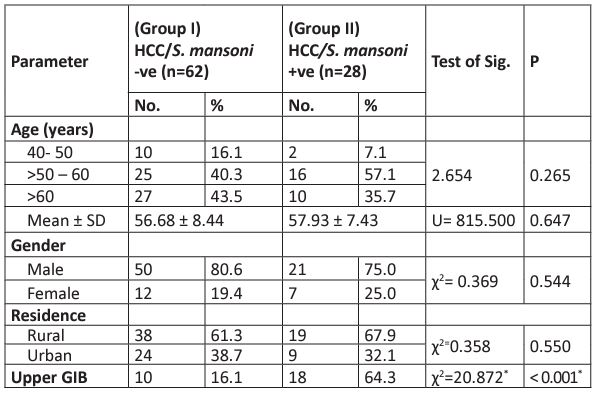
All 90 HCC patients were negative for S. mansoni by Kato-Katz. Based on IHA; 62 HCC/S. mansoni negative patients were classified as group I and 28 S. mansoni positives were in group II. The mean age of all HCC patients was 57.07± 8.12 years, with no significant difference between HCC alone and HCC S. mansoni co-infection. The larger proportion of HCC patients were in the age between 50-60 years among both groups. Regarding gender, HCC was about four times more prevalent in males compared to females; 78.9% (71/90) versus 21.1% (19/90) respectively. Among positive S. mansoni HCC patients: the male to female ratio was 3:1 (75% vs 25%). HCC was more common among patients from rural than urban areas in both groups with no statistically significant difference (p>0.05). As for upper gastrointestinal bleeding, patients with S. mansoni had significantly higher bleeding than those negative for S. mansoni (64.3% versus 16% respectively, p< 0.001) (Table 1).
Table 1: Baseline features of the 90 HCC patients.
U: Mann Whitney test
c2: Chi-square test
p: p-value for comparing between the studied groups, not significant (p>0.01)
* Statistically significant at p≤ 0.05
GIB: gastrointestinal bleeding
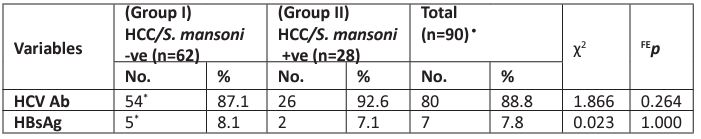
Among the two studied groups, HCV was more prevalent than HBV (88.9% vs 7.8% respectively). Similar patterns of HCV and HBV were observed in both groups; HCV was 87.1% in group I versus 92.6% in group II and HBV was 8.1% vs 7.1% among group I and group II respectively. There was no statistically significant difference between the two studied groups, p> 0.05 (Table 2).
Table 2: Distribution of the two studied groups according to HCV antibodies and HBsAg.
c2: Chi-square test, FE: Fisher Exact. p: p-value for comparing between the studied groups
*Two patients were negative for HCV antibodies and HBsAg and one case had both HCV and HBsAg and they were not included in the calculations
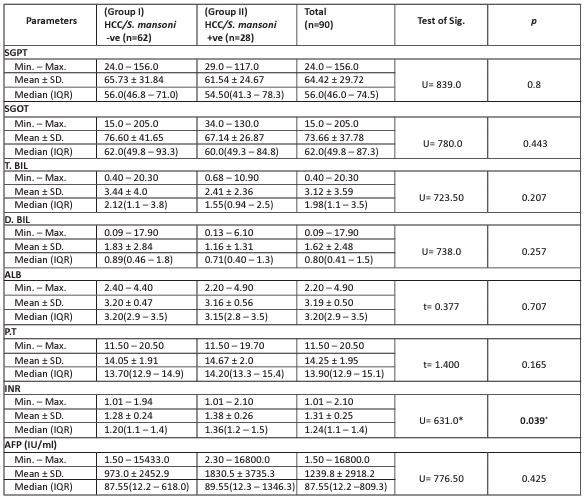
Table 3 revealed that all HCC patients had high liver enzymes levels regarding alanine aminotransferase (SGPT), aspartate aminotransferase (SGOT), total bilirubin (T.BIL), direct bilirubin (D.BIL), albumin (ALB), prothrombin time (P.T) with no statistically significant difference between the two groups. Regarding the international normalization ratio (INR), it was statistically significantly higher in HCC/S. mansoni positives (groups II) compared to HCC/S. mansoni negatives (group I). Mean serum AFP level was elevated among all studied patients (1239.8± 2918). The mean value of AFP in group II (1830.5±3735.3 IU/ml) was higher than that in group I (973.0 IU/ml) yet not significant.
Table 3: Comparison of the laboratory investigation results between the two studied groups.
c2: Chi-square test, FE: Fisher Exact. p: p-value for comparing between the studied groups
SGPT: alanine aminotransferase, SGOT: aspartate aminotransferase, T.BIL: total bilirubin, D.BIL: direct bilirubin, ALB albumin, P.T: prothrombin time, INR: international normalization ratio.
Child-Pugh Scoring System and BCLC among the Two Studied Groups
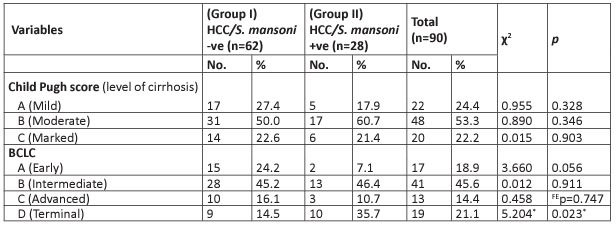
In table 4, based on the Child-Pugh scoring system, a smaller proportion of group II had mild cirrhosis compared to group I, while a greater proportion of group II had moderate cirrhosis with no statistically significant difference. Marked cirrhosis was found in equal proportion in the two groups without any level of significance.
Regarding the BCLC staging system, in class A (early), more cases in group I revealed a higher percentage compared to those in group II (24.2% versus 7.1% respectively) with a borderline statistically significant difference. There was no statistically significant difference between group I and group II in classes B and C. However, HCC/S. mansoni co-infection revealed a higher percentage in class D (terminal) compared to group I with a statistically significant difference (p<0.05).
Table 4: Child-Pugh scoring system and BCLC among the HCC studied groups.
c2: Chi-square test
FE: Fisher Exact
p: p-value for comparing between the studied groups
*: Statistically significant at p≤ 0.05
Discussion
HCC is commonly a disease of infectious origin; consequently, it could be preventable [11]. In the present study, the association of S. mansoni with HBV and/or HCV was investigated as a risk factor for the development of HCC. A total of 90 HCC cases were eligible for this study; they were categorized into HCC patients negative for schistosomiasis (62 patients) and HCC patients positive for schistosomiasis (28 patients).
In this study, the larger proportion of HCC positive for S. mansoni was in the age group 50-60 years with no statistical significance. Abdel-Wahab et al. (2007) [27] and El-Zayadi et al. (2005) [28] revealed different findings and the predominant age was 40-59 years, this was explained by the different localities, the risk of exposure and the study periods. As for gender, HCC was more prevalent in males than females (3:1) in the two groups with no significant difference. Concordant results were obtained by El-Zayadi et al. (2005) and El-Tonsy et al. (2016) concerning gender[28, 29].
Considering residence, the rural areas dwelled patients showed that HCC was 1.5 times higher than those living in urban areas (63% vs 36% respectively) with no statistically significant difference. These findings agreed with those of Abdel-Wahab et al. (2007) [27] and El-Tonsy et al. (2016) [29], which attributed the high prevalence in rural areas to the use of insecticides, pollution, and aflatoxins.
Regarding the clinical data, gastrointestinal hemorrhage in patients with HCC is common and is a major contributor to mortality [30]. The present results revealed that upper GIB was highly significant among positive S. mansoni group compared to the negative one. Consistently, it was reported that schistosomiasis fibrosis is associated with several vascular changes within the host. The peripheral destruction of the portal vein system with the obstruction of some medium-sized branches explains the presence of portal hypertension (one of the S. mansoni infection complications) which leads to upper GIB [31-33].
During the present study period, 88.9% and 7.8% of HCC patients had HCV and HBV correspondingly without statistically significant difference among the two groups, despite the higher proportion of HCC patients showing concomitant HCV and S. mansoni. The development of a vaccine against HBV explains its lower prevalence. Similar results were reported by Abdel-Wahab et al. (2007) , Shaker et al. (2013) and Zampino et. al. (2015)[6, 27, 34]. Regarding, S. mansoni and HCV co-infection, El-Zayadi et al. (2005) [28] and Toda et al.(2015) described that Schistosoma infection may modify the sequence of hepatitis C and lead to more significant complications and faster progression to HCC than patients with no schistosomiasis parasite burden[12]. Instead, Hassan et al. (2001)[35] concluded that HCV infection increases the risk of HCC whereas S. mansoni infection does not. Currently, Egypt succeeded to transform HCV from a health system stigma to a global success story through the 100 million healthy lives initiative launched in October 2018. Hopefully this may lead to lowering the prevalence of HCC.
Regarding the liver enzyme levels (SGPT, SGOT), total bilirubin, direct bilirubin and albumin, they were higher in HCC S. mansoni positives compared to S. mansoni negatives yet not statistically significant. Regarding the INR, there was a statistically significant difference between both groups. The concomitant infection or over infection with HCV in patients with schistosomiasis could be the cause of provocation of liver damage and subsequently reduction in coagulation factors production leading to prolonged prothrombin time. Similarly, (Kamal et al., 2000a) [36] reported more fibrosis in liver biopsies in S. mansoni and HCV concomitant infections compared to HCV mono- infection.
The present results showed that AFP values ranged between 1.5 and 16800 IU/ml in all cases. Although the AFP level in the two studied groups showed no statistical difference, its mean value in the S. mansoni positive group was higher than that in the negative one (1830.5 and 973.0 IU/ml respectively). It was reported that the increased levels in high risk patients provide HCC diagnostic clues. Yet, normal level does not exclude its presence. It was reported that 10-30% of HCC patients have negative AFP expression. On the contrary, many investigators believe that AFP is significant in predicting HCC recurrence and evaluating the biologic features of tumors and treatment regimens [16, 23]. It was pointed out that AFP prevalence among HCC is accompanied by certain controversies and needs novel directions for prospective studies [16, 37].
In the current study, the Child-Pugh classification revealed no significant difference between the two studied groups. However, BCLC classification showed significant statistical difference between the two groups regarding class (D). Therefore, the concomitant association of HCV and schistosomiasis may have increased the severity of liver pathology and progress of HCC.
As for the role of S. mansoni in inducing HCC, cumulating findings of human clinical investigations and experimental research in animal models on a molecular level suggest S. mansoni is potentially associated with HCC. This association was explained by its activation of the proto-oncogenes; c-Jun signal transducer and transcription 3, whose role in liver inflammation and carcinogenesis is well established [1]. This study together with others suggest that chronic inflammation resulting from prolonged parasitic infections may encourage carcinogenesis by three mechanisms; first, chronic inflammation that damages host cell and gene expression by supporting the release of reactive oxygen and nitrogen species; second, insertion of oncogenes into the host genome particularly in the presence of hepatitis B and hepatitis C, stimulation of mitosis and inhibition of tumor suppressors; third, reduction of immune response and induction of immune suppression [11, 12, 28,38].
In conclusion, although in the current study, the co-occurrence of S. mansoni with HCV and HBV quickens hepatic dysplastic changes and accelerates cancer progression yet; these associations are still being sorted out. So, further studies on large cohorts of HCC patients are essential.
Acknowledgments
The authors thank all the staff members of Kafr El Sheikh Liver Disease Research Institute for providing the required data, clinical advice, and unending cooperation.
Conflict of Interest
None declared
References
- Calvisi DF. Schistosoma mansoni and Hepatocellular Carcinoma: Is It All About c-Jun and Signal Transducer and Activator of Transcription 3? Hepatology. 2020; 72(2): 375-378. doi: 10.1002/hep.31392.
- Davis A. Schistosomiasis. In: Cook G, ed. Manson’s Tropical Diseases. 20th ed. London: WB Saunders: 1996; 1413-1456.
- Hu X, Chen R, Wei1Q, Xu X. The Landscape of Alpha Fetoprotein In Hepatocellular Carcinoma: Where Are We? Int J Biol Sci. 2022; 18(2): 536-551. doi: 10.7150/ijbs.64537
- Ohata K, Hamasaki K, Toriyama K, Ishikawa H, Nakao K, Eguchi K (2004) High viral load is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2004; 19(6): 670-675. https://doi.org/10.1111/j.1440-1746.2004.03360.x
- Rashed WM, Kandeil MAM, Mahmoud MO, Ezzat S. Hepatocellular Carcinoma (HCC) in Egypt: A comprehensive overview. J Egypt Natl Canc Inst. 2020; 16; 32(1): 5. doi: 10.1186/s43046-020-0016-x. PMID: 32372179.
- Shaker MK, Abdella HM, Khalifa MO, El Dorry AK. Epidemiological characteristics of hepatocellular carcinoma in Egypt: a retrospective analysis of 1313 cases. Liver Int. 2013; 33(10): 1601-1606. DOI: 10.1111/liv.12209
- El-Tonsy MM, Hussein HM, Helal TES, Tawfik RA, Koriem KM, Hussein HM. Human Schistosomiasis mansoni associated with hepatocellular carcinoma in Egypt: current perspective J Parasit Dis. 2016; 40(3): 976-980. doi: 10.1007/s12639-014-0618-0.
- Barakat RMR. Epidemiology of Schistosomiasis in Egypt: Travel through Time: Review. J Adv Res. 2013; 4(5): 425-432.
- Elbaz T, Esmat G. Hepatic and Intestinal Schistosomiasis. J Adv Res. 2013; 4(5): 445-452. doi: 10.1016/j.jare.2012.12.001
- Strickland GT. Liver disease in Egypt: Hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatol. 2006; 43(5): 915-922.
- El-Tonsy MM, Hussein HM, Helal TES, Tawfik RA, Koriem KM, et al. Schistosoma mansoni infection: Is it a risk factor for development of hepatocellular carcinoma? Acta Tropica. 2013; 128: 542-547. https://doi.org/10.1016/j.actatropica.2013.07.024
- Toda KS, Kikuchi L, Chagas AL, Tanigawa RY, Paranaguá-Vezozzo DC, et al (2015) Hepatocellular Carcinoma Related to Schistosoma mansoni Infection: Case Series and Literature Review. J Clin Transl Hepatol. 2015; 3(4): 260-264. doi: 10.14218/JCTH.2015.00027
- Alboraie M, Youssef N, Sherief AF, Afify S, Wifi MN, et al. Egyptian liver library: An indexed database for liver disease evidence in Egypt. Arab Enterol. 2019; 2(6):109-113. https://doi.org/10.1016/j.ajg.2019.05.004
- Tateyama M, Yatsuhashi H, Taura N, et al. Alpha-fetoprotein above normal levels as a risk factor for the development of hepatocellular carcinoma in patients infected with hepatitis C virus. J Gastroenterol. 2011; 46(1): 92-100.
- Bruno Daniele B, Bencivenga A, Megna AS, Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterol. 2004; 127(5): 108-112. DOI:10.1053/j.gastro.2004.09.023
- Hu X, Chen R, Wei Q, Xu X. The landscape of alpha fetoprotein in hepatocellular carcinoma: Where are we? Int J Biol Sci. 2022; 18(2):536-551. doi: 10.7150/ijbs.64537. PMID: 35002508; PMCID: PMC8741863.
- Pugh RNH, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973; 60(8): 646-649. doi: 10.1002/bjs.1800600817.
- Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010; 30(1): 61-74. doi: 10.1055/s-0030-1247133
- Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, et al.ESMO Guidelines Committee. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018; 29(Suppl 4): iv238-iv255. doi: 10.1093/annonc/mdy308.
- Jelic S, Sotiropoulos GC (2010) Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 21( Suppl 5): 59-64. doi: 10.1093/annonc/mdq166
- Jun CH, Yoon JH, Cho E, Shin SS, Cho SB, et al. Barcelona clinic liver cancer-stage C hepatocellular carcinoma: A novel approach to subclassification and treatment. Medicine (Baltimore). 2017; 96(17): e6745. doi: 10.1097/MD.0000000000006745.
- Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972; 14(6): 397-400.
- Chang TS, Wu YC, Tung SY, Wei KL, Hsieh YY, et al. Alpha-fetoprotein measurement benefits hepatocellular carcinoma surveillance in patients with cirrhosis. Am J Gastroenterol. 2015; 110(6): 836-44; quiz 845. doi: 10.1038/ajg.2015.100.
- Sauzay C, Alexandra Petit A, Bourgeois AM, Barbare JC, Chauffert B, et al. Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma. Clin Chim Acta. 2016; 1; 463:39-44. doi: 10.1016/j.cca.2016.10.006.
- Kirkpatrick LA, Feeney BC. A simple guide to IBM SPSS statistics for version 20.0. Student ed. Belmont, Calif.: Wadsworth, Cengage Learning. 2013.
- Altman GA. Practical statistics for medical research. London, Chapman and Hall. 1992; 404-408.
- Abdel-Wahab M, El-Ghawalby N, Mostafa M, Sultan A, El-Sadany M, et al. Epidemiology of hepatocellular carcinoma in lower Egypt, Mansoura Gastroenterology Center. Hepatogastroenterol. 2007; 54(73): 157-162.
- el-Zayadi AR, Badran HM, Barakat EM, Attia Mel D, Shawky S, et al. Hepatocellular carcinoma in Egypt: a single center study over a decade. World J Gastroenterol. 2005; 11(33): 5193-5198. DOI: 10.3748/wjg.v11.i33.5193
- El-Tonsy MM, Hussein HM, Helal Tel S, Tawfik RA, Koriem KM, et al. Human Schistosomiasis mansoni associated with hepatocellular carcinoma in Egypt: current perspective. J Parasit Dis. 2016; 40(3): 976-980. doi: 10.1007/s12639-014-0618-0.
- Srivastava DN, Gandhi D, Julka PK, Tandon RK. Gastrointestinal hemorrhage in hepatocellular carcinoma: management with transhepatic arterioembolization. Abdom Imaging. 2000; 25: 380-384. DOI: 10.1007/s002610000056
- Johnson P. Malignant tumours of the liver. In B. Bacon, G. O’Grady, A. Di Bisceglie & J. Lake (Eds.), Comprehensive Clinical Hepatology. 2006; 2nd edn: 453–485. Philadelphia, PA: Elsevier Mosby publications.
- Kumar R, Saraswat MK, Sharma BC, Sakhuja P, Sarin SK (2008) Characteristics of hepatocellular carcinoma in India: a retrospective analysis of 191 cases. QJM. 2008; 101(6): 479-485. DOI: 10.1093/qjmed/hcn033
- Andrade ZA. Schistosomiasis and liver fibrosis. Parasite immunol. 2009; 31(11): 656-663. https://doi.org/10.1111/j.1365-3024.2009.01157.x
- Zampino R, Pisaturo MA, Cirillo G, Marrone A, Macera M, et al. Hepatocellular carcinoma in chronic HBV-HCV co-infection is correlated to fibrosis and disease duration. Ann Hepatol. 2015; 14(1): 75-82.
- Hassan MM, Zaghloul AS, El-Serag HB, Soliman O, Patt YZ, et al. The role of hepatitis C in hepatocellular carcinoma: a case control study among Egyptian patients. J Clin Gastroenterol. 2001; 33(2): 123-126. DOI: 10.1097/00004836-200108000-00006.
- Kamal S, Madwar M, Bianchi L, Tawil AE, Fawzy R, et al. Clinical, virological and histopathological features: long-term follow-up in patients with chronic hepatitis C co-infected with S. mansoni. Liver. 2000a: 20(4): 281-289. DOI: 10.1034/j.1600-0676.2000.020004281.x
- Lee CW, Tsai HI, Lee WC, Huang SW, Lin CY, et al. Normal Alpha-Fetoprotein Hepatocellular Carcinoma: Are They Really Normal?. Journal of Clinical Medicine. 2019; 8(10): 1736. doi:10.3390/jcm8101736.
- Abdelghani E, Zerpa R, Iliescu G, Escalante CP. Schistosomiasis and liver disease: Learning from the past to understand the present. Clin Case Rep. 2020; 8(8): 1522-1526. doi: 10.1002/ccr3.2922. PMID: 32884787; PMCID: PMC7455423.